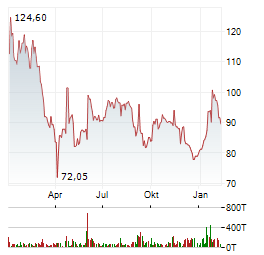
| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
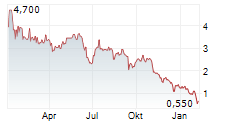
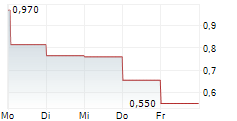
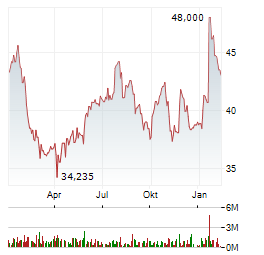
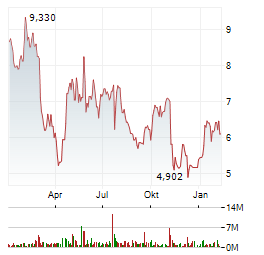
| 0,760 | 0,860 | 16:31 |
Chart-Typ:
Zeitraum:
| Shortseller-Positionen aktuell: freenet, Gerresheimer, Hypoport, Qiagen, Renk, Suss MicroTec, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen |
| Shortseller-Positionen aktuell: freenet, GFT, HelloFresh, Hypoport, Puma, Qiagen, Redcare Pharmacy | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen |
| flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen |
| Evotec: Das Biotech-Drama geht in die nächste Runde | Die Aktie des Hamburger Wirkstoffforschers Evotec versucht erneut den Befreiungsschlag, doch das Misstrauen am Markt ist greifbar. Nach einer beispiellosen Serie von Rückschlägen - von Management-Skandalen... ► Artikel lesen |
| Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen |
| Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen |
| Revolution in der Krebstherapie: Vidac Pharma attackiert den Krebs-Thron der Großen! Warum der kleinere Mitbewerber Riesen wie Bayer und BioNTech die Show stehlen könnte! | Die Welt der Biotechnologie und Pharmazie ist aktuell in Aufruhr, denn technologische Durchbrüche und strategische Neuausrichtungen versprechen eine spannende Zukunft für Anleger. Besonders im Fokus... ► Artikel lesen |
| BioNTech Aktie: Die Wende naht! Golden Cross! | © Foto: 2026 BioNTech SE. Alle Recht vorbehalten.Fast wie in der Serie "Game of Thrones", wo der Winter naht, könnte bei BioNTech die Wende nahen. Nach dem Kursrausch in der Corona-Ära folgte der brutale... ► Artikel lesen |
| BioNTech SE: BioNTech veröffentlicht am 10. März 2026 Ergebnisse für das vierte Quartal und das Geschäftsjahr 2025 und informiert über operativen Fortschritt | MAINZ, Deutschland, 24. Februar 2026 (GLOBE NEWSWIRE) -- BioNTech SE (Nasdaq: BNTX, "BioNTech" oder "das Unternehmen") wird am Dienstag, den 10. März 2026, die Ergebnisse für das vierte Quartal und... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu GENENTA SCIENCE SPA ADR finden Sie auf Wallstreet Online.