| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
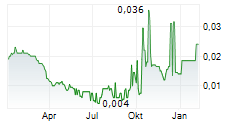
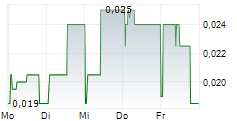
| 0,020 | 0,024 | 11:48 |
Chart-Typ:
Zeitraum:
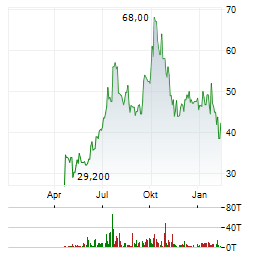
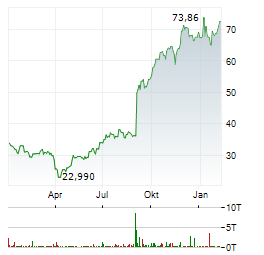
| CRISPR Therapeutics-Aktie: Goldgrube oder Kapitalvernichtung? | Wir sind bekennende GARP-Anhänger (Growth at a Reasonable Price). Nicht aus Prinzip, sondern aus Erfahrung. Wachstum ja - aber bitte nicht um jeden Preis. Wir haben in den letzten zwanzig Jahren genug... ► Artikel lesen |
| CRISPR Therapeutics AG: CRISPR Therapeutics Provides Business Update and Reports Fourth Quarter and Full Year 2025 Financial Results | ZUG, Switzerland and BOSTON, Feb. 12, 2026 (GLOBE NEWSWIRE) -- CRISPR Therapeutics (Nasdaq: CRSP) today reported financial results for the fourth quarter and full year ended December 31, 2025. "As... ► Artikel lesen |
| Morningstar, Inc.: Morningstar Completes Acquisition of CRSP and Extends Relationship with Vanguard | The CRSP integration unites two trusted sources of market insight, reinforcing a shared commitment to transparency, quality, and investor-focused solutions and solidifying Morningstar's position... ► Artikel lesen |
| EU Approves DAWNZERA For Hereditary Angioedema, Says Ionis And Otsuka | TOKYO (dpa-AFX) - Ionis Pharmaceuticals, Inc. (IONS) and Otsuka Pharmaceutical Co., Ltd. on Wednesday said the European Commission has approved Dawnzera to prevent repeated attacks of hereditary... ► Artikel lesen |
| Ionis Pharmaceuticals, Inc.: DAWNZERA (donidalorsen) approved in the European Union for hereditary angioedema (HAE) | Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) and Otsuka Pharmaceutical Co., Ltd. (Otsuka) today announced that the European Commission (EC) has approved DAWNZERA (donidalorsen) in the European Union... ► Artikel lesen |
| Ionis Pharmaceuticals, Inc.: TRYNGOLZA (olezarsen) approved in the European Union for familial chylomicronemia syndrome (FCS) | Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) and Sobi® today announced that TRYNGOLZA® (olezarsen) has been approved in the European Union (EU) as an adjunct to diet in adult patients for the treatment... ► Artikel lesen |
| PacBio Announces Fourth Quarter and Full Year 2025 Financial Results | MENLO PARK, Calif., Feb. 12, 2026 (GLOBE NEWSWIRE) -- PacBio (NASDAQ: PACB) today announced financial results for the quarter and fiscal year ended December 31, 2025. Fourth quarter and full year... ► Artikel lesen |
| PacBio Completes Sale of Short-Read Sequencing Assets | MENLO PARK, Calif., Feb. 02, 2026 (GLOBE NEWSWIRE) -- PacBio (NASDAQ: PACB), a leading developer of high-quality, highly accurate sequencing solutions, today announced the completion of the sale of... ► Artikel lesen |
| PacBio Announces Plans for Collaboration With n-Lorem Foundation and EspeRare to Advance Precision Therapies for Rare Genetic Diseases | MENLO PARK, Calif., Jan. 12, 2026 (GLOBE NEWSWIRE) -- PacBio (NASDAQ: PACB), developer of the world's most advanced sequencing technologies, today announced plans to pursue a strategic collaboration... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu GENFLOW BIOSCIENCES PLC finden Sie auf Wallstreet Online.