Markt oder Branche auswählen:
Chart-Typ:
Zeitraum:
| Dividendenbekanntmachungen (13.11.2025) | Unternehmen ISIN-Code Dividende (Währung) Dividende (EUR) AFFILIATED MANAGERS GROUP INC US0082521081 0,01 USD 0,0086 EUR AIMS APAC REIT SG2D63974620 0,0199 SGD 0,0132 EUR AKOLA GROUP AB LT0000128092 - 0... ► Artikel lesen |
| XFRA CAPITAL ADJUSTMENT INFORMATION - 12.11.2025 | Das Instrument DEVL SG1L01001701 DBS GRP HLDGS SD 1 EQUITY wird cum Kapitalmassnahme gehandelt am 12.11.2025 und ex Kapitalmassnahme am 13.11.2025 The instrument DEVL SG1L01001701 DBS GRP HLDGS SD 1... ► Artikel lesen |
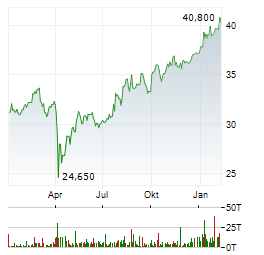
| DBS Group Q3 Net Profit Declines | BRUSSELS/FRANKFURT/PARIS (dpa-AFX) - DBS Group Holdings reported third quarter net profit of S$2.95 billion compared to S$3.03 billion, last year. Reported earnings per share was S$4.12 compared... ► Artikel lesen |

| Maybank, MR DIY, Tenaga Nasional Berhad, Watsons & ZUS Coffee are among the Honoured Winners of the 2025-2026 World Branding Awards in Osaka | The 20th World Branding Awards celebrated the success and achievements of leading brands across National, Regional, and Global categories. In the 2025-2026 edition, over 1,092 brands from 66 countries... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.