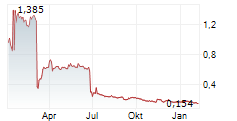
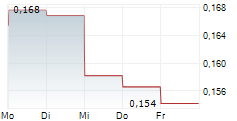
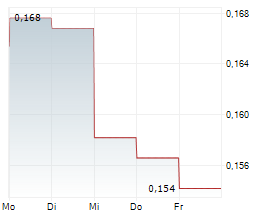
IRLAB has confirmed that clinical candidate IRL757, which has been designed to address apathy in patients with neurodegenerative conditions, will proceed to the next stages of clinical development. The next step will be a safety and efficacy signal finding Phase Ib trial in patients with Parkinson's disease (PD), which will be financially supported by IRLAB's collaboration partner, the McQuade Center for Strategic Research and Development (MSRD) through an initial payment of US$4.5m. The company expects patient enrolment for this Phase Ib study to commence in H225. We note that the decision to advance this programme was based on data from the preceding two Phase I studies for IRL757 and believe that the external recognition highlights the potential of IRLAB's third clinical candidate.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research