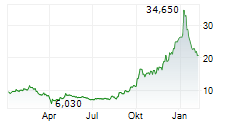
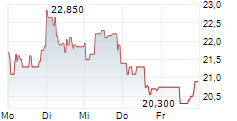
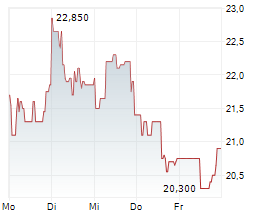
Newron Pharmaceuticals has published its FY24 results, reflecting a rewarding period for its lead clinical candidate, evenamide, which is being developed for treatment-resistant schizophrenia (TRS). Key achievements included licensing agreements for Japan and South Korea ahead of the planned pivotal Phase III trial, expected to launch in Q225. We expect the €44m upfront payment from EA Pharma to provide operational headroom into H126, with further liquidity likely to be injected following a potential US up-listing (planned for early 2026). Reflecting Newron's FY24 results and near-term operational guidance, we adjust our valuation to CHF385.6m or CHF19.3/share (from CHF368.5m or CHF18.5/share previously).Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research