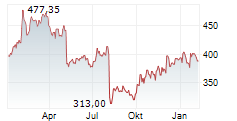
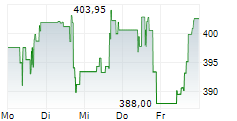
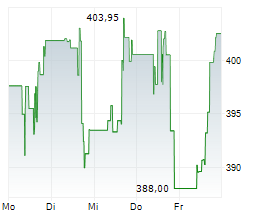
CAMBRIDGE (MASSACHUSETTS) (dpa-AFX) - Vertex Pharmaceuticals (VRTX) announced that the European Commission has approved a label expansion for KAFTRIO (ivacaftor/tezacaftor/elexacaftor) in combination with ivacaftor. The expanded indication now includes cystic fibrosis patients aged 2 years and older with at least one non-class I mutation in the CFTR gene.
As a result of existing KAFTRIO reimbursement agreements in Austria, Denmark, Ireland, Norway and Sweden, and provisions for access in health care systems such as Germany, eligible patients in these countries will have access to the expanded indication of the therapy shortly.
Vertex noted that it will continue to work collaboratively with reimbursement authorities across the European Union to ensure access for all eligible patients, as quickly as possible.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News