THOUSAND OAKS (dpa-AFX) - Sandoz (SDZNY) has initiated an antitrust lawsuit in the United States against Amgen, Inc. (AMGN), accusing the company of maintaining its dominant market position for the blockbuster drug Enbrel (etanercept). This drug, initially approved by the U.S. Food and Drug Administration in 1998, is at the center of the dispute.
Etanercept is a biologic medicine used to treat a range of disabling inflammatory diseases.
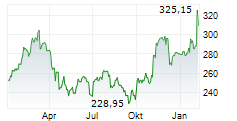
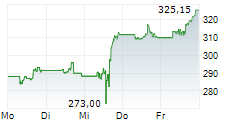
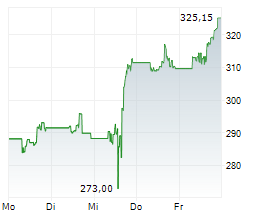
Sandoz alleges that Amgen blocked competition from more cost-effective biosimilar competitors, including Sandoz etanercept biosimilar, Erelzi+ (etanercept-szzs), by unlawfully purchasing and using certain patent rights to entrench its position in the market. In 2024, Enbrel generated $3.3 billion in revenue in the US.
Sandoz received US FDA approval for Erelzi in 2016, the same year the company launched the medicine in Europe.
Sandoz alleged that Amgen is continuing to block entry of this important treatment option for approximately 7.5 million Americans living with chronic inflammatory diseases, including rheumatoid arthritis, psoriasis, psoriatic arthritis, ankylosing spondylitis and juvenile idiopathic arthritis, many of whom could benefit from the cost savings and expanded access resulting from the introduction of high-quality, more-affordable biosimilar options.
Sandoz is seeking an injunction to prevent Amgen from using certain patent rights to block biosimilar competition and allow Sandoz to launch Erelzi as soon as possible. The company is also seeking damages, which could be tripled under applicable laws. The lawsuit was filed in the US District Court for the Eastern District of Virginia.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News