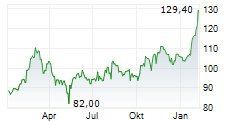
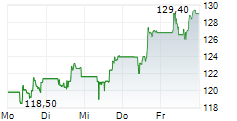
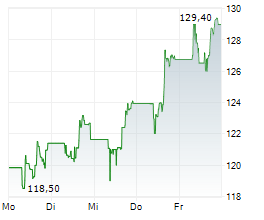
FOSTER CITY (dpa-AFX) - Gilead Sciences, Inc. (GILD) Monday reported positive topline results from the Phase 3 ASCENT-04/KEYNOTE-D19 study evaluating Trodelvy in combination with Merck's Keytruda in patients with triple-negative breast cancer (mTNBC) whose tumors express PD-L1. The study met its primary goal.
In the study, Trodelvy in combination with Keytruda significantly improved progression-free survival (PFS) compared to Keytruda and chemotherapy in patients with mTNBC. Further, the safety profile of Trodelvy plus Keytruda was consistent with the known safety profile of each agent, and no new safety signals were identified with the combination.
Currently, Gilead has three ongoing Phase 3 studies investigating Trodelvy, including the upcoming ASCENT-03 study in 1L mTNBC patients who are not candidates for PD-L1 based therapy, the ASCENT-05 study in patients with early-stage TNBC, and the ASCENT-07 study in patients with HR+/HER2- mBC who have received endocrine therapy. Trodelvy is also being investigated in additional Phase 3 studies in various other cancers including lung and gynecological cancers.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News