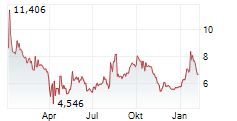
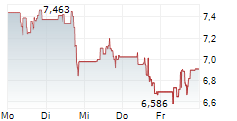
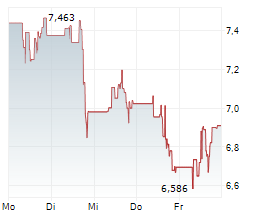
WASHINGTON (dpa-AFX) - Novavax, Inc. (NVAX) announced Wednesday that it recently received formal communication from the U.S. Food and Drug Administration (FDA) in the form of an information request for a postmarketing commitment (PMC) to generate additional clinical data. This is regarding the Biologics License Application (BLA) for Novavax's COVID-19 vaccine.
Novavax said, 'We look forward to engaging with the FDA expeditiously to address the PMC request and move to approval as soon as possible.'
The company had said it believes that the BLA is approvable based on conversations with the FDA, as of the Prescription Drug User Fee Act (PDUFA) date of April 1. The BLA included robust Phase 3 clinical trial data?that showed the vaccine is safe and effective for the prevention of COVID-19.
The company had earlier said it had responded to all of the FDA's information requests and were confident the well-tolerated vaccine represents an important alternative to mRNA COVID-19 vaccines for the U.S.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News