WASHINGTON (dpa-AFX) - Labcorp (LH) Wednesday has announced a significant expansion of its precision oncology offerings with two advanced diagnostic solutions to improve cancer detection and treatment decisions.
These additions include Labcorp Plasma Detect, a blood-based test for assessing recurrence risk in stage III colon cancer patients, and PGDx elio plasma focus Dx, the first FDA-authorized kitted liquid biopsy for pan-solid tumors.
Labcorp Plasma Detect: Monitoring Colon Cancer Recurrence Labcorp Plasma Detect leverages whole-genome sequencing (WGS) to identify circulating tumor DNA (ctDNA), a key marker of molecular residual disease (MRD) and potential cancer recurrence. With a recurrence rate of nearly 30% for stage III colon cancer within five years, this test offers a critical early detection tool for patients at higher risk. The MRD-positive status detected by the test can help guide decisions on further treatment. The assay requires no bespoke panel, delivers results in as few as seven days for follow-up tests, and is currently under evaluation in more than 10 global clinical studies. Initially launched for biopharma use, it is now available for clinical use through an Early Experience Program.
PGDx elio plasma focus Dx: FDA-Authorized Liquid Biopsy for Targeted Therapy The PGDx elio plasma focus Dx is the first liquid biopsy kit of its kind to receive De Novo authorization from the FDA. Designed for use across a range of solid tumors, this tool provides oncologists with a rapid, in-lab method for identifying patients eligible for targeted therapies-all from a single blood draw. Its kitted format allows labs to maintain control over specimens and data, requires only on-site verification for implementation, and delivers results within 4-5 days. The test also integrates seamlessly with PGDx elioT tissue complete, allowing efficient transition from tissue to liquid biopsy workflows.
Looking Ahead Labcorp will showcase data on both tests at the AACR 2025 Annual Meeting, reinforcing its commitment to transforming cancer diagnostics and improving patient outcomes through cutting-edge, personalized solutions.
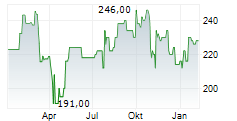
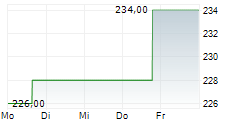
Wednesday, LH closed at $224.14, up 1.77%, and is currently trading flat on the NYSE.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News