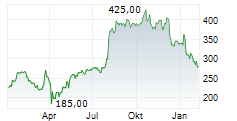
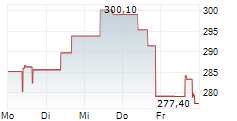
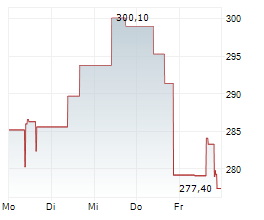
WASHINGTON (dpa-AFX) - Alnylam Pharmaceuticals, Inc. (ALNY) announced that the Committee for Medicinal Products for Human Use of the European Medicines Agency has adopted a positive opinion recommending approval of its RNAi therapeutic vutrisiran for the treatment of wild type or hereditary transthyretin amyloidosis in adult patients with cardiomyopathy. Vutrisiran is currently approved in the European Union under the brand name AMVUTTRA for the treatment of hereditary transthyretin-mediated amyloidosis in adult patients with stage 1 or stage 2 polyneuropathy.
Alnylam said it remains on track to proceed with additional global regulatory submissions for vutrisiran in 2025 and beyond.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News