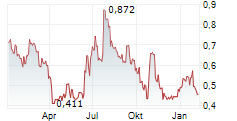
Mendus's Q125 report included no surprises, with the company staying on track to secure large-scale GMP production of vididencel and Phase III readiness by H225, which we see as the most significant upcoming catalysts for the company. Key highlights for the quarter were regulatory endorsements from both the FDA and EMA on the upcoming registrational study for vididencel, as well as first patient enrolment in the Phase II, investigator-sponsored CADENCE study, data from which will contribute to the safety dossier for vididencel, ahead of the Phase III trial. The Q125 operating loss was SEK30.2m, down 14.4% y-o-y, albeit fully attributable to lower R&D costs related to the technology transfer to manufacturing partner NorthX Biologics. Note that this will reverse in the next few quarters and should not affect our FY25 projections or estimates for the cash runway (into early 2026, in line with management guidance). We expect no material change to our valuation from the Q125 results.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research