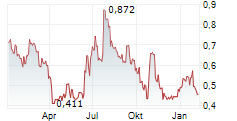
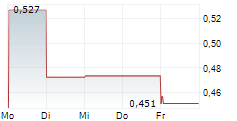
Mendus presented a positive clinical update for its Phase I ALISON trial in ovarian cancer at the 61st Annual American Society of Clinical Oncology conference (ASCO 2025). Importantly, the data demonstrated that patients with high-risk ovarian cancer exhibited stable disease when treated with vididencel, Mendus's proprietary off-the-shelf cancer vaccine, owing to its ability to induce tumour-directed immune responses against antigens implicated in this type of cancer. ALISON is fully recruited and is in the follow-up stage, aiming to assess the longer-term survival benefit. The next readout, based on two years of follow-up, is anticipated in Q425, potentially representing an upcoming catalyst for investor attention.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research