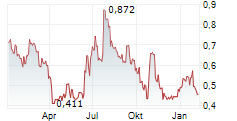
Mendus's Q125 results came in as expected, with management confirming that vididencel (in acute myeloid leukaemia, AML) is on track to be pivotal-stage ready from H225. Recent highlights include regulatory endorsements from the FDA and EMA of Mendus's plans for a registrational trial for vididencel in AML, as well as enrolment of the first patient in the Phase II CADENCE trial (sponsored and conducted by the Australasian Leukaemia and Lymphoma Group, ALLG). Beyond AML, vididencel is also being assessed in the Phase I ALISON trial (in ovarian cancer, OC); interim data have been encouraging and the next update is expected in May 2025. Mendus also recently presented a DCOne platform update, offering potential to expand its value proposition in the OC and solid tumour space. With a strengthened leadership team, with Tariq Mughal joining as CMO in May 2025, and a robust net cash position of SEK83.9m, which should provide a runway to Q126, we believe Mendus is well positioned to advance its clinical programmes. Following the Q125 results, our valuation adjusts to SEK1.94bn or SEK38.4/share (from SEK1.90bn or SEK37.8/share).Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research