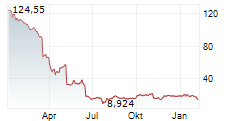
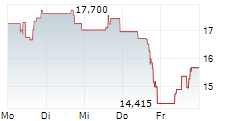
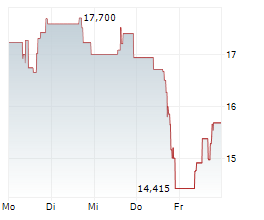
WASHINGTON (dpa-AFX) - Sarepta Therapeutics, Inc. (SRPT), Tuesday said that the Japanese Ministry of Health, Labour, and Welfare (MHLW) has approved Elevidys for the treatment of Duchenne muscular dystrophy under the conditional and time-limited approval pathway in Japan.
Elevidys is approved for individuals ages 3- to less than 8-years-old, who do not have any deletions in exon 8 and/or exon 9 in the DMD gene, and who are negative for anti-AAVrh74 antibodies. This is the first global approval to include individuals younger than 4 years of age.
The approval is based on the efficacy and safety data for Elevidys, which includes muscle health and longer-term functional results from the Elevidys clinical programs, including the two-year data from the global Phase 3 Embark clinical trial.
'For nearly a decade, Sarepta has been a leader in advancing the treatment of Duchenne muscular dystrophy through innovative treatment options. With the approval of ELEVIDYS in Japan, children who are living with this rare, fatal disease, one marked by progressive muscle deterioration and weakness, now have an additional treatment option,' said Louise Rodino-Klapac, Sarepta's head of research and development and chief scientific officer. 'Sarepta is committed to supporting our partners so we can bring this treatment to additional people with Duchenne around the world who urgently need a therapy that potentially addresses the root cause of disease.'
The conditional and time-limited approval pathway in Japan provides for marketing authorization in Japan for up to seven years for innovative medicines to treat serious conditions. To be eligible for the pathway, certain additional criteria must also be met, including early clinical trial results that have demonstrated significant efficacy and safety.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News