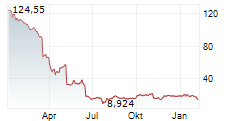
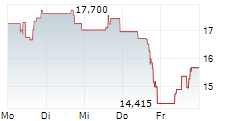
WASHINGTON (dpa-AFX) - Sarepta Therapeutics, Inc. (SRPT) Wednesday said that the UK's Medicines & Healthcare products Regulatory Agency (MHRA) has given clearance to continue dosing in the ENVISION Study of its gene therapy Elevidys for the treatment of Duchenne Muscular Dystrophy.
ENVISION is a Phase 3 study of Elevidys in non-ambulatory and older ambulatory individuals with Duchenne.
In the U.S., Elevidys is approved for the treatment of Duchenne Muscular Dystrophy in patients aged 4 and above.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News