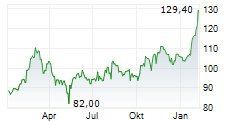
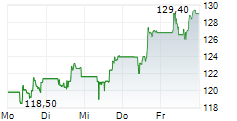
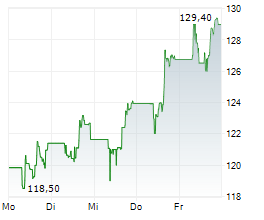
FOSTER CITY (dpa-AFX) - Gilead Sciences, Inc. (GILD) Friday announced positive topline results from the Phase 3 ASCENT-03 study of Trodelvy in patients with first-line metastatic triple-negative breast cancer (mTNBC).
The study met its primary goal showing statistically significant and clinically meaningful improvement in progression-free survival (PFS) compared to chemotherapy in patients with first-line mTNBC
The safety profile of Trodelvy in the ASCENT-03 study was consistent with prior studies, and no new safety signals were identified.
Phase 3 studies of Trodelvy across HER2- breast cancer including the ASCENT-07 trial in patients with HR+/HER2- mBC who have received endocrine therapy, and the ASCENT-05 study in patients with early-stage TNBC (eTNBC) are underway. The drug is also being evaluated in additional phase 3 studies in various types of tumors, including lung and gynecologic cancers.
'Together with the recently announced positive results from the ASCENT-04 study evaluating Trodelvy plus Keytruda in patients with previously untreated PD-L1+ metastatic TNBC, Trodelvy now has the potential to be the backbone treatment for all patients across first-line mTNBC,' the company said in a statement.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News