WASHINGTON (dpa-AFX) - The U.S. Department of Health and Human Services has notified Moderna (MRNA) that it will terminate funding for the late-stage development of pre-pandemic influenza vaccines, along with the right to purchase them. Meanwhile, Moderna said it will explore alternatives for late-stage development and manufacturing of the H5 program consistent with the company's strategic commitment to pandemic preparedness.
After a rigorous review, HHS reportedly concluded that further investment in Moderna's H5N1 mRNA vaccine is neither scientifically nor ethically justifiable. HHS Communications Director Andrew Nixon reportedly stated that the decision was based on careful assessment of available data and broader public health consideration.
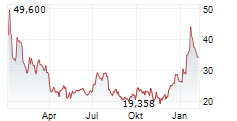
In January 2025, the U.S. Department of Health and Human Services or HHS said that it would provide approximately $590 million to Moderna to accelerate the development of mRNA-based pandemic influenza vaccines and enhance mRNA platform capabilities.
In 2024, the company received $176 million from HHS toward its vaccine efforts.
On Wednesday, Moderna announced positive interim data from a Phase 1/2 clinical study evaluating the safety and immunogenicity of its investigational pandemic influenza vaccine, mRNA-1018, in approximately 300 healthy adults aged 18 years and older. The interim results focus on a vaccine candidate targeting the H5 avian influenza virus subtype.
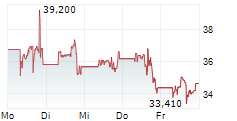
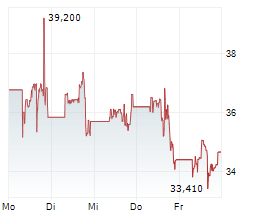
The company had previously expected to advance the program to late-stage development with the U.S. Department of Health and Human Services (HHS); however, today Moderna received notice that HHS will terminate the award for the late-stage development and right to purchase pre-pandemic influenza vaccines.
'While the termination of funding from HHS adds uncertainty, we are pleased by the robust immune response and safety profile observed in this interim analysis of the Phase 1/2 study of our H5 avian flu vaccine and we will explore alternative paths forward for the program,' said Stéphane Bancel, Chief Executive Officer of Moderna.
According to the company, the investigational vaccine was generally well-tolerated, with no dose-limiting tolerability concerns observed. Most solicited adverse reactions were Grade 1 or 2 and did not increase significantly with number of doses or between first and second doses. Further data is expected to be submitted for presentation at an upcoming scientific meeting.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News