WASHINGTON (dpa-AFX) - Neurocrine Biosciences, Inc. (NBIX), Monday announced data from the Phase 4 KINECT-PRO open-label study, evaluating patient-reported outcomes on the use of INGREZZA capsules in a tardive dyskinesia patient population reflective of real-world clinical practice.
The study included a four-week screening period, a 24-week treatment period during which participants received 40 mg of INGREZZA once-daily for the first four weeks, followed by flexible dosing of 40 mg, 60 mg or 80 mg once-daily based on individual treatment needs and a two-week safety follow-up period.
The findings, presented at 2025 Psych Congress Elevate, show robust and clinically meaningful improvements in patients' physical functioning, social interactions and emotional well-being.
The company noted that individuals with milder uncontrolled movement severity also demonstrated negative impact by their tardive dyskinesia at baseline , and meaningful improvements across all severity levels.
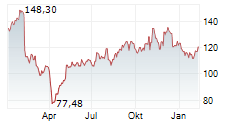
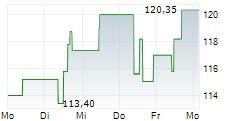
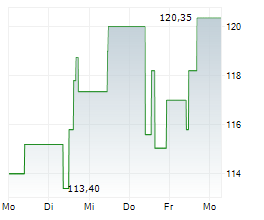
Currently, Neurocrine's stock is trading at $122.01, down 0.82 percent on the Nasdaq.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News