WASHINGTON (dpa-AFX) - Sarepta Therapeutics (SRPT), Wednesday announced that the FDA has designated its rAAVrh74 viral vector used in the investigational gene therapy SRP-9003 for limb-girdle muscular dystrophy R4 as a platform technology.
This recognition underscores the vector's reproducibility and adaptability across multiple gene therapy programs. With this designation, Sarepta can leverage existing rAAVrh74 data to streamline IND, NDA, and BLA filings, potentially accelerating development timelines. SRP-9003 delivers a full-length beta-sarcoglycan transgene via the MHCK7 promoter, targeting skeletal, diaphragm, and cardiac muscles.
The FDA's platform technology program aims to improve efficiency in rare disease drug development by permitting sponsors to build on prior platform data for manufacturing, safety, and quality.
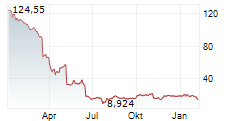
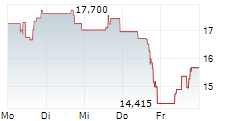
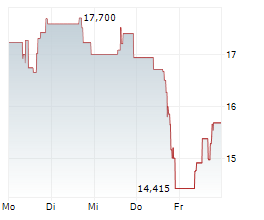
SRPT is currently trading at $39.34, up $0.46 or 1.19 percent on the Nasdaq.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News