FOSTER CITY (dpa-AFX) - The U.S. Food and Drug Administration Tuesday announced that it has placed a clinical hold on Gilead Sciences' (GILD) investigational HIV trials combining GS-1720 and GS-4182.
This action follows identification of a safety signal declines in CD4+ T-cell and absolute lymphocyte counts-in some participants receiving the combo. Trial investigators have been notified. Neither agent is approved anywhere, and Gilead is working with regulators to address the findings and determine a path forward.
This hold affects two Phase 2/3 WONDERS studies and three Phase 1 trials but does not impact Gilead's other long-acting oral or injectable HIV regimens under clinical or preclinical evaluation. Patient safety remains Gilead's top priority.
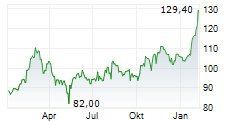
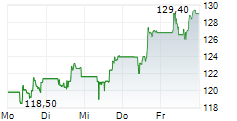
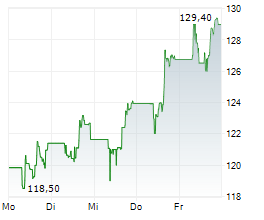
GILD is currently trading at $110.72, down $2.28 or 2.02 percent on the Nasdaq.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News