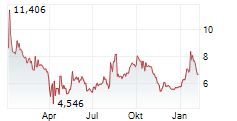
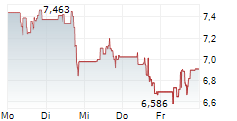
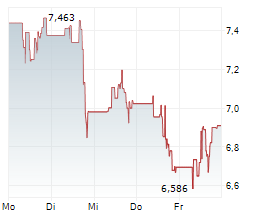
WASHINGTON (dpa-AFX) - Novavax, Inc. (NVAX) reported results of the initial cohort of COVID-19-Influenza Combination and stand-alone trivalent hemagglutinin nanoparticle seasonal influenza Phase 3 trial that showed both the COVID-19-Influenza Combination and flu vaccine candidates induced immune responses similar to licensed comparators Nuvaxovid and Fluzone HD, respectively.
'Both our combination and stand-alone flu vaccine candidates induced robust immune responses and were well tolerated. This data set adds to findings from our Phase 2 trial and will help inform discussions with potential partners,' said Ruxandra Draghia-Akli, Executive Vice President and Head of Research and Development, Novavax.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News