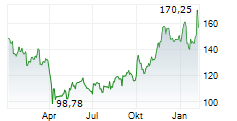
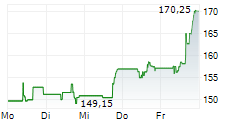
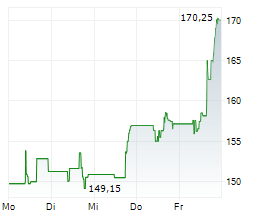
WESTON (dpa-AFX) - UCB (UCB) and Biogen Inc. (BIIB) presented additional detailed results from the Phase 3 PHOENYCS GO study evaluating dapirolizumab pegol (DZP), a novel Fc-free anti-CD40L drug candidate. In the study, dapirolizumab pegol demonstrated significant clinical improvements in disease activity in people living with moderate-to-severe systemic lupus erythematosus (SLE).
According to the company, dapirolizumab pegol showed consistent improvements in fatigue, a common and debilitating symptom of systemic lupus erythematosus (SLE).
At Week 48, more individuals receiving dapirolizumab pegol experienced no or low disease activity compared to standard of care with differences observed as early as Week 12.
The company noted that the safety profile of dapirolizumab pegol was generally favorable. The safety results were consistent with previous dapirolizumab pegol studies and with that in study participants with systemic lupus erythematosus receiving an immunomodulator.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News