WASHINGTON (dpa-AFX) - Moderna Inc. (MRNA) announced that the U.S. Food and Drug Administration has approved its respiratory syncytial virus (RSV) vaccine, mRESVIA (mRNA-1345), for preventing lower respiratory tract disease (LRTD) caused by RSV in adults aged 18-59 who are at increased risk.
The approval expands the previous indication of mRESVIA, which was approved in May 2024 for adults aged 60 years and older.
The company intends to have mRESVIA available for both younger adults at increased risk (ages 18-59) and older adults (ages 60+) in the U.S. for the 2025-2026 respiratory virus season.
The company noted that the approval was supported by results from Moderna's Phase 3 study, which evaluated the safety and immunogenicity of mRESVIA in adults aged 18-59 with underlying health conditions.
The vaccine was generally well-tolerated, and the most commonly reported solicited adverse reactions were injection site pain, fatigue, headache, myalgia and arthralgia.
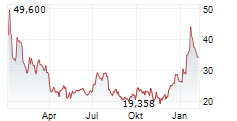
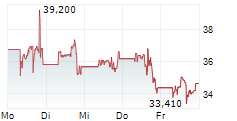
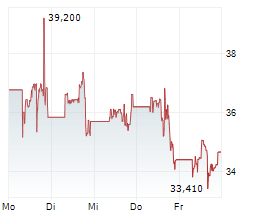
MRNA closed Thursday's regular trading at $27.35 down $0.40 or 1.44%. But in the after-hours trading the stock gained $0.10 or 0.36%.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News