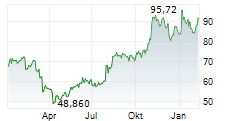
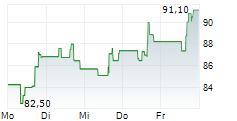
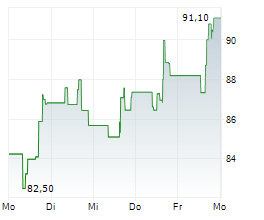
WASHINGTON (dpa-AFX) - Incyte (INCY) announced that the U.S. Food and Drug Administration has approved Monjuvi (tafasitamab-cxix), a humanized Fc-modified cytolytic CD19-targeting monoclonal antibody, in combination with rituximab and lenalidomide for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL).
The company noted that the milestone represents the second approved indication for Monjuvi in the United States.
The company noted that patients with relapsed or refractory follicular lymphoma achieved significantly improved progression-free survival with Monjuvi in combination with rituximab and lenalidomide in the Phase 3 registration trial.
In July 2020, Monjuvi in combination with lenalidomide received FDA approval for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, including DLBCL arising from low grade lymphoma, and who are not eligible for autologous stem cell transplant (ASCT). Tafasitamab is also being evaluated as a therapeutic option in an ongoing pivotal trial for first-line DLBCL.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News