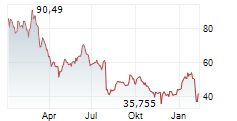
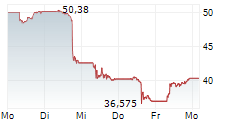
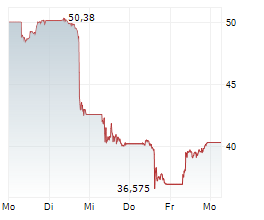
WASHINGTON (dpa-AFX) - Novo Nordisk (NVO) announced that the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion to update the Ozempic (once-weekly semaglutide) label, reflecting data from the STRIDE trial on functional outcomes in peripheral artery disease.
Peripheral artery disease or PAD is a manifestation of atherosclerotic cardiovascular disease (ASCVD) where a build-up of fatty deposits in the artery walls restricts blood supply to muscles, which can cause debilitating symptoms, physical limitations and poor quality of life.
Novo Nordisk expects the European Commission to implement the label update within approximately two months. Novo Nordisk has also filed for a label expansion of Ozempic in the US, and a decision is expected in last quarter of 2025.
In addition, Novo Nordisk said it has filed for a label expansion for Rybelsus with the EMA and FDA. This could potentially make Rybelsus the first and only oral GLP-1 RA with proven cardiovascular (CV) benefits. A decision is also expected in the second half of 2025.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News