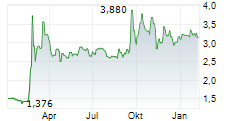
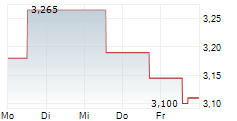
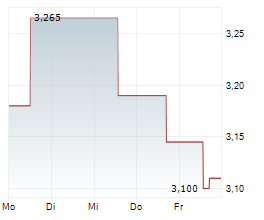
Oryzon Genomics has submitted the Phase III clinical trial protocol to the FDA for its lysine-specific demethylase 1 (LSD1) inhibitor, vafidemstat, in borderline personality disorder (BPD), marking a major step towards developing its lead programme as a potential first-in-class therapy for aggression in BPD. The primary endpoint will be the previously defined STAXI-2 Trait Anger score, where vafidemstat had demonstrated statistically significant improvement in the previous Phase IIb study. The key secondary endpoint will be the score on the clinician-rated Modified Overt Aggression Scale (OAS-M). The study design incorporates guidance from the FDA and input from US psychiatric key opinion leaders (KOLs), which we believe significantly derisks the Phase III programme. We expect FDA clearance in Q325, with Phase III to commence in 2026, potentially under a partnering agreement. Our valuation remains unchanged following the announcement.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research