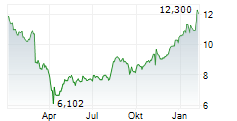
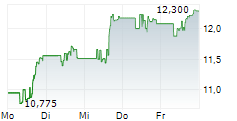
WASHINGTON (dpa-AFX) - Theravance Biopharma, Inc. (TBPH) announced that Viatris Inc. has secured regulatory approval from China's National Medical Products Administration for YUPELRI inhalation solution, the first once-daily nebulized long-acting muscarinic antagonist approved for maintenance treatment of chronic obstructive pulmonary disease in China.
The approval triggers a one-time $7.5 million milestone from Viatris to Theravance, which is expected to be received in third quarter, 2025. Theravance Biopharma is also eligible for further sales-based milestones of up to $37.5 million and tiered royalties of 14% to 20% on net sales in China.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News