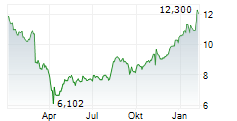
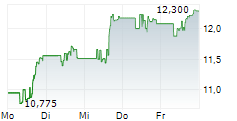
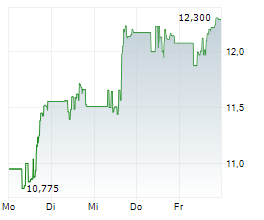
WASHINGTON (dpa-AFX) - Viatris (VTRS) announced the FDA has approved Iron Sucrose Injection, USP, an intravenous iron replacement product used to treat iron deficiency anemia in adult and pediatric patients with chronic kidney disease. The FDA granted Viatris a competitive generic therapy designation for iron sucrose 100 mg/5 mL and 200 mg/10 mL strengths.
Viatris Chief R&D Officer Philippe Martin said, 'The first FDA approval of a generic iron sucrose is an important advancement for patients with CKD and iron deficiency anemia and a testament to Viatris' advanced technical and manufacturing capabilities. This complex product was developed in-house, and after a number of years working closely with the FDA we are pleased to accomplish this important milestone.'
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News