COPENHAGEN (dpa-AFX) - Novo Nordisk (NVO) is broadening patient access to its FDA-approved weight management drug, Wegovy (semaglutide) 2.4 mg, through a new partnership with WeightWatchers starting July 1, 2025.
As part of this collaboration, CenterWell Pharmacy will manage prescription fulfillment and delivery through NovoCare Pharmacy. The initiative integrates Wegovy access with WeightWatchers' lifestyle support system, aiming to streamline care for patients managing chronic obesity.
Dave Moore, Executive Vice President of U.S. Operations at Novo Nordisk, noted the collaboration aligns with evolving patient preferences for care delivery. He highlighted WeightWatchers' decades-long, science-based approach to weight management and praised the synergy between both organizations' commitment to long-term health improvement. He added that Novo Nordisk is encouraged by its ongoing partnerships with Ro and LifeMD and remains focused on expanding access to its FDA-approved therapies.
Alongside this, Novo Nordisk is launching a new savings initiative: a $299 introductory offer for self-paying patients who are new to Wegovy through NovoCare, valid from July 1 to July 31, 2025. This follows the expiration of the earlier $199 offer on June 30, 2025. Eligible patients who used the $199 offer between May 22 and June 30 will qualify for one $299 fill in July. Thereafter, the monthly price for self-pay patients will be $499.
Patients can access the savings via Wegovy.com or through integrated telehealth providers including WeightWatchers, Ro, and LifeMD. CenterWell Pharmacy will remain the dispensing partner for these offers.
Novo Nordisk also issued a strong warning against compounded 'semaglutide' drugs, which are illegal and potentially dangerous. The FDA has linked severe illnesses and deaths to such unapproved knockoffs, many of which involve illicit ingredients sourced from unregulated manufacturers in China. Novo Nordisk reports that over half of Chinese suppliers distributing 'semaglutide' to the U.S. in 2024-2025 are not authorized to do so, even in China. The company has initiated nearly 120 lawsuits across 34 states and pledges continued legal action and regulatory cooperation to protect patients from unsafe alternatives.
Obesity Context: Obesity is a complex, chronic disease influenced by biological, genetic, environmental, and social factors, not just willpower. Affecting roughly 40% of U.S. adults, it poses significant health risks and burdens healthcare systems.
About Wegovy (semaglutide) Injection 2.4 mg: Wegovy is an injectable prescription medication designed to assist with weight loss and reduce cardiovascular risk when used alongside diet and exercise. It is approved for adults and adolescents (12+ years) with obesity, or overweight individuals with related health conditions.
Important Safety Information: Wegovy may cause serious side effects, including potential thyroid tumors or cancer, particularly in individuals with a family history of medullary thyroid carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Other serious risks include pancreatitis, gallbladder issues, kidney problems, low blood sugar (especially in patients with diabetes), severe gastrointestinal problems, increased heart rate, depression or suicidal thoughts, and complications during surgery involving sedation. Common side effects may include nausea, vomiting, diarrhea, stomach pain, headache, fatigue, and general digestive discomfort.
Patients should disclose all health conditions and medications to their healthcare provider before using Wegovy. The drug should not be used in children under 12 or those allergic to semaglutide.
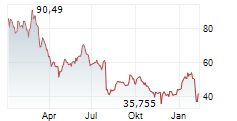
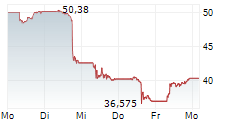
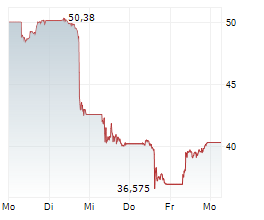
NVO currently trades at $67.41 or 0.09% higher on the NYSE.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News