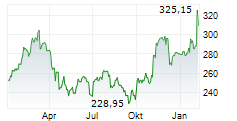
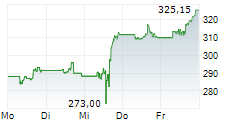
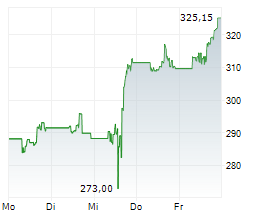
THOUSAND OAKS (dpa-AFX) - Biopharmaceutical company Amgen, Inc. (AMGN) announced Monday that the Phase 3 FORTITUDE-101 clinical trial evaluating first-line bemarituzumab plus chemotherapy (mFOLFOX6) met its primary endpoint of overall survival (OS) at a pre-specified interim analysis.
Bemarituzumab plus chemotherapy demonstrated a statistically significant and clinically meaningful improvement in OS as compared to placebo plus chemotherapy in people living with unresectable locally advanced or metastatic gastric or gastroesophageal junction (G/GEJ) cancer with FGFR2b overexpression and who are non-HER2 positive.
FGFR2b overexpression was defined as 2+/3+ staining in ?10% of tumor cells by centrally performed immunohistochemistry (IHC) testing.
The company said these first positive top-line results of an FGFR2b targeted monoclonal antibody from the Phase 3 FORTITUDE-101 study mark a meaningful advance in the development of effective targeted therapy for gastric cancer.
While ocular events were consistent with the Phase 2 experience and observed in both arms, they occurred with greater frequency and severity in the Phase 3 bemarituzumab arm.
FORTITUDE-101 was conducted with the support of Zai Lab Ltd. (ZLAB), which holds co-development and commercialization rights for bemarituzumab for mainland China, Hong Kong, Macau, and Taiwan.
A Phase 3 study of bemarituzumab plus chemotherapy and nivolumab is also ongoing in patients with first-line gastric cancer, with a data readout anticipated in the second half or 2025.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News