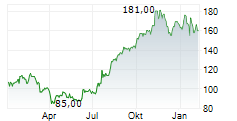
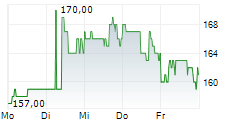
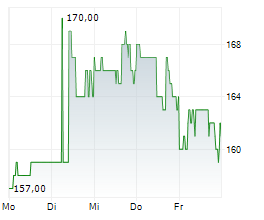
WASHINGTON (dpa-AFX) - Ligand Pharmaceuticals Inc. (LGND) and Pelthos Therapeutics Inc. announced Wednesday the closing of the previously announced merger agreement with Channel Therapeutics Corp. (CHRO), resulting in the launch of Pelthos as an independent, publicly traded biopharmaceutical company.
Under the deal, CHRO Merger Sub Inc., a unit of Channel, merged with and into LNHC, Inc., a unit of Ligand, with LNHC surviving as a Channel subsidiary.
The combined company will operate under the name Pelthos Therapeutics Inc. and will commence trading on the NYSE American exchange under the new ticker symbol PTHS on July 2.
Further, Pelthos plans to launch ZELSUVMI for the treatment of Molluscum contagiosum infections in July 2025.
The companies in mid-April signed a definitive merger agreement to combine Ligand's subsidiaries, Pelthos and LNHC, with Channel's CHRO Merger.
Concurrent with the deal closure, Pelthos raised $50.1 million of equity capital, including a private placement from a group of strategic investors led by Murchinson. The Investor Group invested $32 million and Ligand invested $18 million in the combined company, respectively.
The capital is being invested into Pelthos Series A Convertible Preferred Stock and Common Stock. It includes cancellation of around $18.8 million in bridge capital that was advanced to Pelthos by several of the Investors, including Ligand, since the beginning of 2025 to support the commercial launch of ZELSUVMI.
Ligand is entitled to a 13% royalty on worldwide net sales of ZELSUVMI.
Pelthos plans to initially focus on the launch and commercialization of ZELSUVMI (berdazimer) topical gel, 10.3%, for the treatment of molluscum contagiosum infections in adults and pediatric patients one year of age and older.
ZELSUVMI is an FDA-designated novel drug and the first and only prescription medication approved for the treatment of molluscum, a poxvirus and one of the most common skin infections, that can be administered at home by parents, patients, and caregivers.
In addition, Pelthos is continuing to evaluate the path forward for its existing NaV 1.7 development programs for the treatment of various types of chronic pain, acute and chronic eye pain, and post-surgical nerve blocks.
Todd Davis, CEO of Ligand, said, 'This moment is the result of the vision, hard work, and unwavering commitment of our internal team, who developed the commercial platform to help bring ZELSUVMI, a novel product addressing a significant unmet need, to market this summer. Ligand recognized the potential value of ZELSUVMI before it was approved, during a time when others did not, and we are proud to bring this impactful treatment to market through our special situations initiatives.'
For More Such Health News, visit rttnews.com
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News