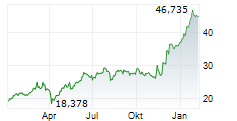
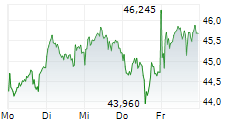
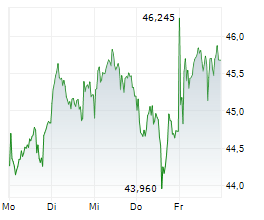
LEVERKUSEN (dpa-AFX) - Bayer announced that the FDA has approved finerenone or Kerendia for the treatment of adult patients with heart failure and a left ventricular ejection fraction of ?40%. Finerenone is now indicated to reduce the risk of cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in adult patients with heart failure with LVEF of ?40%.
The company said the new indication approval follows the FDA's Priority Review designation and is based on positive results from the Phase III FINEARTS-HF study, which is part of the ongoing MOONRAKER program.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News