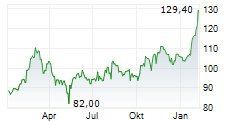
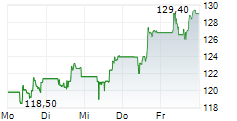
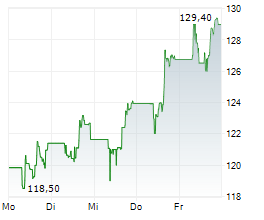
FOSTER CITY (dpa-AFX) - Gilead Sciences, Inc. (GILD) Monday announced that Gilead researchers and collaborators will present new Phase 3 Purpose trial data at IAS 2025 showing that twice-yearly lenacapavir (Yeztugo) was effective and well tolerated among a broad range of populations who need or want pre-exposure prophylaxis (PrEP) for HIV prevention, including pregnant and lactating women, adolescents and young people, and supports lenacapavir dosing recommendations for people in special situations, such as those taking medication to treat tuberculosis (TB) and other conditions.
Researchers will also present new quantitative and qualitative data showing that participants in both Phase 3 Purpose trials indicated a preference for twice-yearly PrEP injections over daily oral medication.
The new data, from the company's pivotal Phase 3 Purpose 1 (NCT04994509) and Purpose 2 (NCT04925752) trials that assessed the efficacy and safety of twice-yearly Yeztugo for PrEP, will be presented via poster sessions and during a Yeztugo-dedicated oral session at the International AIDS Society (IAS) 2025, the 13th IAS Conference on HIV Science in Kigali, Rwanda on Thursday, July 17.
'It's a thrill to be back in Kigali with so many of the community, advocacy and research partners who helped make PURPOSE the most intentionally inclusive HIV prevention trial program ever conducted,' said Moupali Das, Vice President of Clinical Development, HIV Prevention & Pediatrics at Gilead Sciences. 'As the first and only twice-yearly PrEP option, Yeztugo continues to demonstrate efficacy and tolerability among diverse populations, and we're excited to highlight new data on this breakthrough HIV prevention option here at IAS 2025.'
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News