Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics (the "Company") (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today announced a financing through a capital increase reserved to an existing shareholder by the issuance of new shares with warrants attached, for a total gross amount of EUR 499,999.92 (excluding the future net proceeds related to the exercise of the warrants) (the "Reserved Offering"). The subscription price for one ABSA is EUR 0.17 (the "Offering Price
This follows the approximately EUR 4 million financing announced on July 1, 2025 (the "Early July Financing"). The Offering Price of the ABSA and the terms and conditions of the warrants, including their exercise price, are identical to those included in the Early July Financing.
"We are delighted to receive this additional support from an existing shareholder, as we continue to pursue GenSight's strategic direction and LUMEVOQ's potential," commented Laurence Rodriguez, Chief Executive Officer of GenSight Biologics. "This supplementary funding, combined with our recent EUR 4 million financing, strengthens our position as we advance toward the anticipated opening of the French AAC program in Q4 2025 and prepare for our global Phase III RECOVER study.
The New Shares (as defined below) represent less than 30% of the total number of the Company's ordinary shares already admitted to trading on Euronext Paris. The admission to trading of the New Shares on Euronext Paris is therefore exempted from the obligation to prepare a prospectus.
Use of Proceeds
The proceeds of the Reserved Offering will supplement the funds raised in the Early July Financing. Together, they are intended to be used principally to finance the continued development of LUMEVOQ, the Company's most advanced drug candidate, currently in Phase III. In particular, the funds raised will help ensure the Company's operational continuity, support the finalization of manufacturing transfer activities and secure the preparations for the anticipated opening of compassionate use access in France as well as the initiation of the Phase III study RECOVER. The balance will be used to meet certain financial obligations of the Company. This will extend the Company's cash runway from mid-July 2025 before the Early July Financing to early-October 2025 initially and now to mid-October 2025.
"This additional financing provides valuable flexibility as we execute our operational roadmap through October 2025," noted Jan Eryk Umiastowski, Chief Financial Officer of GenSight Biologics. "We believe the support of UPMC Enterprises reflects the attractiveness of our investment proposition. We remain focused on achieving key regulatory milestones that will establish a sustainable revenue pathway, beginning with the AAC program.
Terms and Conditions of the Reserved Offering
The Reserved Offering, for a total of EUR 499,999.92 (share issue premium included), was carried out through the issuance of 2,941,176 ABSA (as defined below) via a capital increase without shareholders' preferential subscription rights reserved to a category of persons satisfying determined characteristics, pursuant to Article L. 225-138 of the French Commercial Code, through the issuance of new shares of a per value of EUR 0.025 (the "New Shares"), to which are attached 1 warrant for 1 new share (the "Warrants", together with the New Shares, the "ABSA" and with the new shares of the Company resulting from the exercise of the Warrants, the "Warrant Shares
The launch of the Reserved Offering was decided on July 16, 2025 by the Chief Executive Officer, pursuant to the delegation of competence granted to her by the Company's board of directors (the "Board of Directors") on June 19 and June 30, 2025. The Board of Directors acted pursuant to the delegation of competence granted to it under the 24th resolution of the Company's shareholders on May 13, 2025 (the "General Meeting
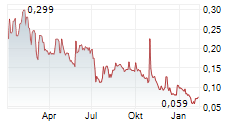
The EUR 0.17 issue price on one ABSA represents a facial premium of EUR 0.0492 to the closing price of the GenSight shares on Euronext Paris on the day of the determination of the issue price, i.e., EUR 0.1208 on July 16, 2025. The exercise price of the Warrants is EUR 0.21.
The Warrants will be exercisable at any time from their issue date until July 21, 2030.
The exercise of a Warrant will give the right to subscribe to one (1) Warrant Share (the "Exercise Ratio"), it being specified that this Exercise Ratio may be adjusted following any transactions carried out by the Company on its share capital or reserves, as from the issuance date of the Warrants, in order to maintain the rights of the Warrants' holders.
Impact of the Reserved Offering on the Company's Shareholding
Following the issuance of the New Shares, the Company's total share capital will be EUR 3,891,473.45 (composed of 155,658,938 ordinary shares). If all the Warrants are exercised, and thus all the Warrant Shares issued, the Company's total share capital will be EUR 3,965,002.85 (composed of 158,600,114 ordinary shares).
Please see sections 19.1.3 and 19.1.4 of the Company's 2024 Universal Registration Document and the press release dated July 1, 2025 relating to the Early July Financing for a description of the securities issued by GenSight and giving access to its capital.
For illustration purposes, the impact of the issuance of the New Shares and the Warrant Shares on the ownership of a shareholder holding 1% of the Company's share capital prior to the Reserved Offering and not subscribing to it, is as follows:
Ownership interest (in %) | ||
On a non-diluted basis | On a diluted basis1 | |
Prior to the issue of the New Shares | 1.00% | 0.59% |
Following the issue of the New Shares | 0.98% | 0.57% |
Following the issue of the New Shares and the Warrant Shares from the exercise of all the Warrants | 0.96% | 0.57% |
Impact of the Reserved Offering on Shareholders' Equity
For illustration purposes, the impact of the issuance of the New Shares and the Warrant Shares on the Company's equity per share (calculation made on the basis of the Company's shareholders' equity as reflected in the consolidated financial statements as of December 31, 2024, adjusted for the capital increase of March 7, 2025, the conversion of 631,560 Heights convertible bonds into 1,930,195 new shares on April 1, 2025, in accordance with the amendment dated June 27, 2024 to the convertible bond agreement, and the capital increase of July 1, 2025) is as follows:
Share of equity per share (in euros) | ||
On a non-diluted basis | On a diluted basis2 | |
Prior to the issue of the New Shares | (0.14) | 0.051 |
Following the issue of the New Shares | (0.14) | 0.054 |
Following the issue of the New Shares and the Warrant Shares from the exercise of all the Warrants | (0.13) | 0.054 |
Evolution of the Shareholding Structure following the Reserved Offering
To the Company's knowledge, immediately prior to completion of the Reserved Offering, the breakdown of the Company's share capital was as follows:
Shareholders | Shareholding (non-diluted) | Shareholding (diluted)3 | ||||
Number of shares and voting rights | % of share capital and voting rights | Number of shares and voting rights | % of share capital and voting rights | |||
5% Shareholders | ||||||
Sofinnova4 | 35,795,627 | 23.4 | 53,106,527 | 20.4 | ||
Invus5 | 21,512,773 | 14.1 | 30,535,967 | 11.7 | ||
UPMC Enterprises6 | 10,158,364 | 6.7 | 12,487,477 | 4.8 | ||
Heights7 | 14,900,613 | 9.8 | 70,347,580 | 27.1 | ||
BPI | 3,877,591 | 2.5 | 5,355,501 | 2.1 | ||
Directors and Officers | 517,002 | 0.3 | 2,388,335 | 0.9 | ||
Employees | 352,500 | 0.2 | 392,500 | 0.2 | ||
Other shareholders (total) | 65,603,292 | 43.0 | 85,398,945 | 32.8 | ||
Total | 152,717,762 | 100.00 |
| 260,012,832 | 100.00 |
|
To the Company's knowledge, immediately after the completion of the Reserved Offering and the issuance of the New Shares, the breakdown of the Company's share capital will be as follows:
Shareholders | Shareholding (non-diluted) | Shareholding (diluted) | ||||
Number of shares and voting rights | % of share capital and voting rights | Number of shares and voting rights | % of share capital and voting rights | |||
5% Shareholders | ||||||
Sofinnova | 35,795,627 | 23.0 | 53,106,527 | 20.0 | ||
Invus | 21,512,773 | 13.8 | 30,535,967 | 11.5 | ||
UPMC Enterprises | 13,099,540 | 8.4 | 18,369,829 | 6.9 | ||
Heights | 14,900,613 | 9.6 | 70,347,580 | 26.5 | ||
BPI | 3,877,591 | 2.5 | 5,355,501 | 2.0 | ||
Directors and Officers | 517,002 | 0.3 | 2,388,335 | 0.9 | ||
Employees | 352,500 | 0.2 | 392,500 | 0.1 | ||
Other shareholders (total) | 65,603,292 | 42.1 | 85,398,945 | 32.1 | ||
Total | 155,658,938 | 100.0 |
| 265,895,184 | 100.0 |
|
To the Company's knowledge, after the completion of the Reserved Offering and the issuance of the New Shares and all Warrant Shares upon exercise of all Warrants, the breakdown of the Company's share capital will be as follows:
Shareholders | Shareholding (non-diluted) | Shareholding (diluted) | ||||
Number of shares and voting rights | % of share capital and voting rights | Number of shares and voting rights | % of share capital and voting rights | |||
5% Shareholders | ||||||
Sofinnova | 35,795,627 | 22.6 | 53,106,527 | 20.0 | ||
Invus | 21,512,773 | 13.6 | 30,535,967 | 11.5 | ||
UPMC Enterprises | 16,040,716 | 10.1 | 18,369,829 | 6.9 | ||
Heights | 14,900,613 | 9.4 | 70,347,580 | 26.5 | ||
BPI | 3,877,591 | 2.4 | 5,355,501 | 2.0 | ||
Directors and Officers | 517,002 | 0.3 | 2,388,335 | 0.9 | ||
Employees | 352,500 | 0.2 | 392,500 | 0.1 | ||
Other shareholders (total) | 65,603,292 | 41.4 | 85,398,945 | 32.1 | ||
Total | 158,600,114 | 100.0 |
| 265,895,184 | 100.0 |
|
Admission to Trading of the New Shares and the Warrants
Settlement-delivery of the Reserved Offering and the admission of the New Shares to trading on the regulated market of Euronext Paris are expected on July 21, 2025.
The New Shares and the Warrant Shares will be subject to the provisions of the Company's by-laws and will be assimilated to existing shares upon final completion of the Reserved Offering. They will bear current dividend rights and will be admitted to trading on the same listing line as the Company's existing shares under the same ISIN code FR0013183985 SIGHT.
The Warrants are expected to be admitted to trading on the Euronext Growth market in Paris ("Euronext Growth Paris") on July 21, 2025 on the same warrant listing line as the Company's existing warrants under the same ISIN code FR0014010IB4/SIGBS.
The Warrant Shares will be admitted to trading on Euronext Paris as they are issued following the exercise of the corresponding warrants.
Lock-up Commitments
The benefit of the lock-up commitments agreed by the Company (45 days) and directors and officers of the Company (60 days) has been extended to UPMC Enterprises.
Indicative Timetable
July 16, 2025 | Decision of the Chief Executive Officer setting the terms and conditions of the Reserved Offering. |
July 17, 2025 | Publication of this press release. |
July 18 or 21, 2025 | Publication of the Euronext notice of admission of the New Shares to trading on Euronext Paris. |
July 21, 2025 | Settlement-delivery of the ABSAs Detachment of the Warrants Start of trading of the New Shares on Euronext Paris. Admission of the Warrants on Euronext Growth Paris |
Risk Factors
The Company draws the attention of the public to the risk factors relating to the Company and its business described in its 2024 Universal Registration Document, as updated in the Information Document made available in connection with the Early July Financing, which are available free of charge on the Company's website (https://www.gensight-biologics.com/)
In addition, the main risks specific to securities are as follows:
- The existing shareholders who do not participate in the Reserved Offering will see their shareholding in the share capital of GenSight diluted, and this shareholding may also be further diluted in the event of the exercise of the Warrants, as well as in the event of new securities transactions.
- The volatility and liquidity of GenSight shares could fluctuate significantly. The market price of the Company's shares may fluctuate and fall below the subscription price of the shares issued in the context of the Reserved Offering. The sale of Company shares may occur on the secondary market, after the Reserved Offering, and have a negative impact on the Company share price.
About GenSight Biologics S.A.
GenSight Biologics S.A. is a clinical-stage biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders. GenSight Biologics' pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics, to help preserve or restore vision in patients suffering from blinding retinal diseases. GenSight Biologics' lead product candidate, GS010 (lenadogene nolparvovec) is in Phase III in Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease that leads to irreversible blindness in teens and young adults. Using its gene therapy-based approach, GenSight Biologics' product candidates are designed to be administered in a single treatment to each eye by intravitreal injection to offer patients a sustainable functional visual recovery.
Disclaimer
Not for release, directly or indirectly, in or into the United States of America, Canada, Australia, Japan or South Africa. This press release and the information contained herein do not contain or constitute an offer to subscribe or purchase, or the solicitation of an order to purchase or subscribe, for securities in the United States of America or in any other jurisdiction where such an offer or solicitation would be unlawful. The securities referred to herein have not been and will not be registered under the Securities Act, or under the securities laws of any state or other jurisdiction of the United States of America, and may not be offered or sold in the United States of America except pursuant to an exemption from, or in a transaction not subject to, the registration requirements of the Securities Act and in compliance with the securities laws of any state or any other jurisdiction of the United States. GenSight does not intend to make a public offering of the securities in the United States of America.
The distribution of this press release may be subject to legal or regulatory restrictions in certain countries. Persons in possession of this press release should inform themselves of and observe any local restrictions. The information contained herein is subject to change without notice.
Forward-Looking Statements
This press release contains forward-looking statements. All statements, other than statements of historical facts, included in this press release are forward-looking statements. These forward-looking statements include, but are not limited to, statements regarding the completion expected proceeds and anticipated use of proceeds of the Reserved Offering; the anticipated cash runway of the Company; and future expectations, plans and prospects of the Company. Words such as "anticipates," "believes," "expects," "intends," "projects," and "future" or similar expressions are intended to identify forward-looking statements. These forward-looking statements are subject to the inherent uncertainties in predicting future results and conditions and no assurance can be given that the proposed securities offering discussed above will be consummated on the terms described or at all. Completion of the proposed Reserved Offering and the terms thereof are subject to numerous factors, many of which are beyond the control of the Company, including, without limitation, market conditions, failure of customary closing conditions and the risk factors and other matters set forth in the filings the Company makes with the Autorité des marchés financiers from time to time. The Company expressly disclaims any obligation to update any forward-looking statements, whether because of new information, future events or otherwise, except as may be required by law.
1 The number of shares contained in the table includes 107,295,070 shares that may be issued by the Company further to the exercise of the remaining share warrants, founders share warrants, free shares and stock options outstanding, including the share warrants issued as part of the Early July Financing.
2 Idem.
3 Idem.
4 Sofinnova Partners: French management company located at 7-11 boulevard Haussmann, 75009 Paris, France, which manages Sofinnova Crossover I SLP.
5 Invus: a Bermudian company located at Clarendon House, 2 Church Street, Hamilton HM 11 Bermuda. Pursuant to the provisions of Article L. 233-9 I, 4° bis of the French Commercial Code, Invus has stated that they hold 6,360,058 shares of GENSIGHT BIOLOGICS S.A. as a result of holding "contracts for differences" ("CFDs") maturing on January 3, 2034, covering an equivalent number of GENSIGHT BIOLOGICS S.A. shares, to be settled in cash.
6 UPMC Enterprises: a non-profit organization located at 6425 Penn Avenue, Suite 200, Pittsburgh, Pennsylvania, United States of America.
7 Heights Capital: a Cayman Islands exempted company located at PO Box 309GT, Ugland House South Church Street, George Town Grand Cayman, Cayman Islands.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250716271672/en/
Contacts:
GenSight Biologics
Chief Financial Officer
Jan Eryk Umiastowski
jeumiastowski@gensight-biologics.com