Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
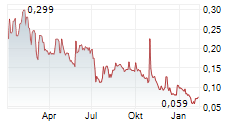
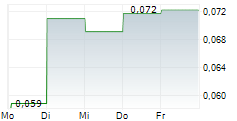
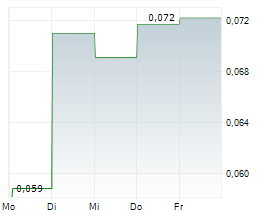
GenSight Biologics (the "Company") (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, announced that the closing of its EUR 499,999.92 (share issue premium included) financing (the "Reserved Offering"), which was announced on July 17, 2025, took place today, July 22, 2025.
The Reserved Offering resulted in the issuance of 2,941,176 ABSA (as defined below) today via a capital increase without shareholders' preferential subscription rights reserved to a category of persons satisfying determined characteristics, pursuant to Article L. 225-138 of the French Commercial Code, through the issuance of new shares of a par value of EUR 0.025 (the "New Shares"), to which are attached 1 warrant for 1 new share (the "Warrants", together with the New Shares, the "ABSA
The New Shares were admitted to trading on Euronext Paris today, July 22, 2025 (ISIN FR0013183985/SIGHT). The Warrants were also admitted to trading on the Euronext Growth market in Paris ("Euronext Growth Paris") today (ISIN FR0014010IB4/SIGBS).
Risk factors
The Company draws the attention of the public to the risk factors relating to the Company and its business described in its 2024 Universal Registration Document, as updated in the Information Document made available in connection with the EUR 4 million financing announced on July 1st, 2025, both of which are available free of charge on the Company's website (https://www.gensight-biologics.com/)
About GenSight Biologics S.A.
GenSight Biologics S.A. is a clinical-stage biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders. GenSight Biologics' pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics, to help preserve or restore vision in patients suffering from blinding retinal diseases. GenSight Biologics' lead product candidate, GS010 (lenadogene nolparvovec) is in Phase III in Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease that leads to irreversible blindness in teens and young adults. Using its gene therapy-based approach, GenSight Biologics' product candidates are designed to be administered in a single treatment to each eye by intravitreal injection to offer patients a sustainable functional visual recovery.
Disclaimer
Not for release, directly or indirectly, in or into the United States of America, Canada, Australia, Japan or South Africa. This press release and the information contained herein do not contain or constitute an offer to subscribe or purchase, or the solicitation of an order to purchase or subscribe, for securities in the United States of America or in any other jurisdiction where such an offer or solicitation would be unlawful. The securities referred to herein have not been and will not be registered under the Securities Act, or under the securities laws of any state or other jurisdiction of the United States of America, and may not be offered or sold in the United States of America except pursuant to an exemption from, or in a transaction not subject to, the registration requirements of the Securities Act and in compliance with the securities laws of any state or any other jurisdiction of the United States. GenSight does not intend to make a public offering of the securities in the United States of America.
The distribution of this press release may be subject to legal or regulatory restrictions in certain countries. Persons in possession of this press release should inform themselves of and observe any local restrictions. The information contained herein is subject to change without notice.
Forward-Looking Statements
This press release contains forward-looking statements. All statements, other than statements of historical facts, included in this press release are forward-looking statements. These forward-looking statements include, but are not limited to, statements regarding the completion expected proceeds and anticipated use of proceeds of the Reserved Offering; the anticipated cash runway of the Company; and future expectations, plans and prospects of the Company. Words such as "anticipates," "believes," "expects," "intends," "projects," and "future" or similar expressions are intended to identify forward-looking statements. These forward-looking statements are subject to the inherent uncertainties in predicting future results and conditions and no assurance can be given that the proposed securities offering discussed above will be consummated on the terms described or at all. Completion of the proposed Reserved Offering and the terms thereof are subject to numerous factors, many of which are beyond the control of the Company, including, without limitation, market conditions, failure of customary closing conditions and the risk factors and other matters set forth in the filings the Company makes with the AMF from time to time. The Company expressly disclaims any obligation to update any forward-looking statements, whether because of new information, future events or otherwise, except as may be required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250722823073/en/
Contacts:
GenSight Biologics
Chief Financial Officer
Jan Eryk Umiastowski
jeumiastowski@gensight-biologics.com