| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 0,086 | 0,092 | 04.03. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 18.02. | GenSight Biologics S.A.: GenSight Biologics Bolsters Regulatory Leadership in US and Europe with Two Senior Appointments | 284 | Business Wire | Regulatory News:
GenSight Biologics ("GenSight Biologics" or the "Company") (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing... ► Artikel lesen | |
| 10.02. | GenSight Biologics S.A.: The 15-20 National Hospital and GenSight Biologics Announce the Treatment of the First Patient in the GS010/LUMEVOQ REVISE Study | 340 | Business Wire | First of 14 planned patients for the dose-ranging study approved by the ANSM in December 2025
Clinical study marks continuing partnership between the 15-20 Hospital and GenSight Biologics... ► Artikel lesen | |
| GENSIGHT BIOLOGICS Aktie jetzt für 0€ handeln | |||||
| 29.01. | GenSight Biologics S.A.: GenSight Biologics Announces the Results of its Extraordinary General Meeting of January 28, 2026 | 850 | Business Wire | Regulatory News:
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal... ► Artikel lesen | |
| 08.01. | GenSight Biologics Reports Cash Position as of December 31, 2025, and Provides Business Updates | 366 | Business Wire | Cash position amounted to €2.4 million as of December 31, 2025
Successful closing of fundraising worth nearly €2.9 million on January 7, 2026
Payments for early access treatments... ► Artikel lesen | |
| 07.01. | GenSight Biologics Announces Closing of Fundraising Worth Nearly EUR 2.9 Million | 331 | Business Wire | Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics (the "Company") (Euronext: SIGHT... ► Artikel lesen | |
| 29.12.25 | GenSight Biologics Announces Successful Fundraising Amounting to Nearly €2.9 Million | 395 | Business Wire | Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics ("GenSight" or the "Company") (Euronext:... ► Artikel lesen | |
| 22.12.25 | GenSight Biologics S.A.: GenSight Biologics Announces Regulatory Authorization for Early Access Treatment with GS010/LUMEVOQ in Israel | 476 | Business Wire | Israel's Ministry of Health authorizes individual patients early access treatment with GS010/LUMEVOQ, a candidate gene therapy for the treatment of ND4-LHON.
Bilateral injection expected... ► Artikel lesen | |
| 22.12.25 | GenSight Biologics S.A.: GenSight Biologics Announces the Granting of Compassionate Use Authorization (CUA/AAC) for GS010/LUMEVOQ in France | 467 | Business Wire | French medicines agency ANSM has granted authorization for named patient early access program for GS010/LUMEVOQ, a gene therapy indicated for the treatment of ND4-LHON.
Regulatory News:
GenSight... ► Artikel lesen | |
| 02.12.25 | GenSight Biologics Announces Regulatory Approval for GS010/LUMEVOQ REVISE Dose-Ranging Study in France | 362 | Business Wire | French medicines agency ANSM and Ethics Committee authorize the dose-ranging study investigating two doses of candidate product for the treatment of ND4-LHON.
Approval of the study protocol... ► Artikel lesen | |
| 13.11.25 | GenSight Biologics Announces Closing of Fundraising Worth Nearly EUR 2 Million | 455 | Business Wire | Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics (the "Company") (Euronext: SIGHT... ► Artikel lesen | |
| 10.11.25 | GenSight Biologics S.A.: GenSight Biologics Announces Successful Fundraising Amounting to Nearly €2 Million | 312 | Business Wire | Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics ("GenSight" or the "Company") (Euronext:... ► Artikel lesen | |
| 30.10.25 | GenSight Biologics Announces Regulatory Authorizations for Individual Patient Expanded Access Treatment with GS010/LUMEVOQ in the US | 533 | Business Wire | FDA authorization and Institutional Review Board (IRB) approval followed by QP release for expanded access treatment of one patient in the US
Treatment scheduled in November 2025 at the... ► Artikel lesen | |
| 07.10.25 | GenSight Biologics Reports Cash Position as of September 30, 2025 | 380 | Business Wire | Cash position amounted to €0.6 million as of September 30, 2025
Successful closing of fundraising worth nearly EUR3.7 million on October 1, 2025
Cash position amounted to €3.6 million... ► Artikel lesen | |
| 01.10.25 | GenSight Biologics S.A.: GenSight Biologics Announces Closing of Fundraising Worth Nearly EUR 3.7 Million | 539 | Business Wire | Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics (the "Company") (Euronext: SIGHT... ► Artikel lesen | |
| 29.09.25 | GenSight Biologics Reports Interim Financial Results for the First Half of 2025 | 518 | Business Wire | Optimized cash management results in 8.3% reduction in operating cash outflow compared to the same period in 2024
Cash runway extended to late Q4 2025 following post-closing financing
... ► Artikel lesen | |
| 26.09.25 | GenSight Biologics S.A.: GenSight Biologics Announces Successful Fundraising Amounting to Nearly EUR 3.7 Million | 385 | Business Wire | Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics (the "Company") (Euronext: SIGHT... ► Artikel lesen | |
| 28.08.25 | GenSight Biologics S.A.: GenSight Biologics Postpones Release of 2025 Half-Year Financial Results | 492 | Business Wire | Regulatory News:
GenSight Biologics ("GenSight Biologics" or the "Company") (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing... ► Artikel lesen | |
| 22.07.25 | GenSight Biologics Announces the Closing of Approximately €500,000 of Additional Financing | 468 | Business Wire | Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics (the "Company") (Euronext: SIGHT... ► Artikel lesen | |
| 17.07.25 | GenSight Biologics Announces Publication on Predictors of Final Visual Outcome in Patients Treated with LUMEVOQ Gene Therapy | 437 | Business Wire | Better baseline visual acuity and thicker baseline optical coherence tomography (OCT) parameters predict better final visual outcome
Patients treated during dynamic phase achieve better... ► Artikel lesen | |
| 17.07.25 | GenSight Biologics Announces an Additional Financing Amounting to Approximately EUR 500,000 from Existing Shareholder | 529 | Business Wire | Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics (the "Company") (Euronext: SIGHT... ► Artikel lesen |
1 2 Weiter >>
32 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 89,10 | -0,11 % | BioNTech Aktie: Die Wende naht! Golden Cross! | © Foto: 2026 BioNTech SE. Alle Recht vorbehalten.Fast wie in der Serie "Game of Thrones", wo der Winter naht, könnte bei BioNTech die Wende nahen. Nach dem Kursrausch in der Corona-Ära folgte der brutale... ► Artikel lesen | |
| EVOTEC | 5,652 | +6,56 % | Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen | |
| BB BIOTECH | 50,50 | +0,60 % | BB Biotech: Gleich mehrere Zukäufe - spannende News erwartet | Im Schlussquartal 2025 hat die Schweizer Biotechgesellschaft BB Biotech einige Veränderungen am Portfolio vorgenommen. Bereits kurz nach dem Kauf wurde bei zwei Werten bereits eine Übernahme angekündigt:... ► Artikel lesen | |
| MEDIGENE | 0,035 | -11,56 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 40,875 | +0,43 % | Shortseller-Positionen aktuell: freenet, Gerresheimer, Hypoport, Qiagen, Renk, Suss MicroTec, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| MODERNA | 49,585 | -0,17 % | Moderna: Kombi-Impfung für Grippe und Covid erhält Zulassung | Gute Nachrichten für Moderna: Die EU-Arzneimittelbehörde EMA hat grünes Licht gegeben für den ersten Kombi-Impfstoff gegen Corona und Grippe. Der Wirkstoff solle für Menschen ab 50 Jahren zugelassen... ► Artikel lesen | |
| PAION | 0,099 | +24,25 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WURDE STATTGEGEBEN | Dem Mistrade-Antrag wurde stattgegeben. Folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wurden aufgehoben:ISIN Datum Zeit Volumen PreisDE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 4,756 | +0,46 % | Valneva-Aktie: Die letzte Chance | Für den französischen Impfstoffentwickler Valneva entscheidet sich 2026 ein großer Teil der Investmentstory. Die Aktie wird derzeit fast ausschließlich durch die Erwartung der Phase-3-Daten zum Lyme-Borreliose-Impfstoff... ► Artikel lesen | |
| AMGEN | 326,85 | +0,31 % | Amgen EVP Sold $20.77M In Company Stock | ||
| EPIGENOMICS | 1,000 | +4,17 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,530 | +0,34 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 330,30 | +0,67 % | Jefferies reiterates Buy on Stryker stock, keeps $465 target | ||
| BIOGEN | 160,15 | +1,59 % | Biogen Inc.: The New England Journal of Medicine Publishes First Data to Demonstrate the Potential for Disease Modification in Dravet Syndrome | -Results show that targeting the underlying cause of Dravet syndrome with zorevunersen may improve outcomes for people with this rare, devastating genetic neurodevelopmental disease-
-Data support... ► Artikel lesen | |
| BIOFRONTERA | 2,730 | +3,41 % | Paradigmenwechsel in der onkologischen Dermatologie: Vidac Pharma als Innovator, was machen Almirall und Biofrontera? | Die onkologische Dermatologie steht an der Schwelle zu einer Revolution, die das bisherige Verständnis der Krebsbehandlung grundlegend verändern könnte. Der globale Markt für die Behandlung der Aktinischen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 3,020 | -2,58 % | HEIDELBERG PHARMA AG unter Beobachtung |
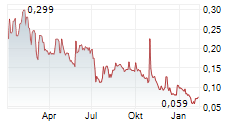
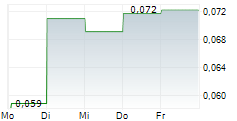
Das Unternehmen GENSIGHT BIOLOGICS SA kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell +25,49 % bei einem Aktienkurs von 0,096 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu GENSIGHT BIOLOGICS SA finden Sie auf Wallstreet Online.