WASHINGTON (dpa-AFX) - The U.S. Food and Drug Administration announced that it has placed Sarepta Therapeutics' (SRPT) investigational gene therapy trials for limb-girdle muscular dystrophy on clinical hold following three patient deaths potentially linked to the therapies. The decision stems from new safety concerns indicating that participants may face an unreasonable and significant risk of illness or injury. The FDA also revoked Sarepta's platform technology designation.
The FDA requested that Sarepta voluntarily halt all shipments of ELEVIDYS, but the company declined to comply.
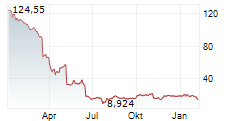
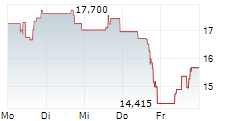
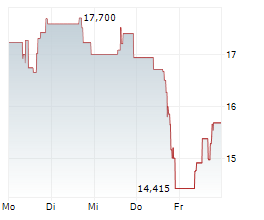
SRPT closed Friday regular trading at $14.07 down $7.89 or 35.94%. In after hours trading, the stock further dopped $0.70 or 5.01%.
In a separate press release, Sarepta confirmed that on Friday it received an informal request from the FDA to voluntarily halt shipments of ELEVIDYS, a gene therapy for Duchenne muscular dystrophy, in the U.S. The company became aware of this potential action around the same time the public did, via media reports.
As a precaution, Sarepta has paused shipments of ELEVIDYS for non-ambulant patients while it works with the FDA to update the product label and assess an enhanced immunosuppression protocol aimed at reducing the risk of acute liver failure. Shipments to ambulant patients will continue, as current data show no new safety concerns for that group, the company said.
According to the company, a recent death of a 51-year-old non-ambulant patient occurred during a Phase 1 trial for SRP-9004, a different investigational therapy intended for treating Limb-Girdle Muscular Dystrophy (LGMD Type 2D). The patient was not treated with ELEVIDYS, and dosing for the SRP-9004 trial had concluded before the incident.
The company notified the FDA of the serious adverse event on June 20 and reported the death on July 3, 2025, as required by law.
Meanwhile, the FDA said on Friday that the three deaths appear to have been a result of acute liver failure in individuals treated with Elevidys or investigational gene therapy using the same AAVrh74 serotype that is used in Elevidys. One of the fatalities occurred during a clinical trial conducted under an investigational new drug application for the treatment of Limb Girdle Muscular Dystrophy.
The FDA said it is continuing to investigate the risk of acute liver failure with serious outcomes, including those such as hospitalization and death, following gene therapies using Sarepta's AAVrh74 Platform Technology, and the need for further regulatory actions.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News