WESTON (dpa-AFX) - Biogen Inc. (BIIB), Friday announced that the Committee for Medicinal Products for Human Use of the European Medicines Agency has backed marketing authorization for ZURZUVAE for the treatment of postpartum depression in adults following childbirth.
The committee's decision is based on the SKYLARK study, where ZURZUVAE met the primary endpoint by demonstrating a significant mean reduction from baseline in the 17-item Hamilton Rating Scale for Depression total score.
Additionally, the study met all secondary endpoints, with significant reduction in depressive symptoms seen as early as Day 3 and sustained through Day 45 compared to placebo.
Moving ahead, the recommendation will be reviewed by the European Commission for marketing authorization in the European Union with a final decision expected in the third quarter of 2025.
If approved by European Commission, ZURZUVAE will be the first treatment authorized within the European Union specifically indicated to treat depressive symptoms for women with postpartum depression.
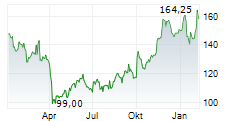
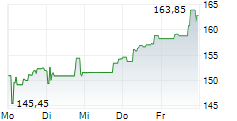
In the pre-market hours, BIIB is trading at $132.59 on the Nasdaq.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News