SOUTH SAN FRANCISCO (dpa-AFX) - The U.S. Food and Drug Administration is investigating the death of an 8-year-old boy who received Elevidys, a gene therapy developed by Sarepta Therapeutics for Duchenne muscular dystrophy. The death occurred on June 7, 2025. In response, the FDA requested-and received-a voluntary suspension of product distribution while it investigates safety concerns. However, Sarepta Therapeutics stated that the patient's death was deemed unrelated to treatment with ELEVIDYS.
Elevidys is an adeno-associated virus vector-based gene therapy using Sarepta Therapeutics, Inc.'s AAVrh74 Platform Technology for the treatment of Duchenne muscular dystrophy. The product is administered as a single intravenous dose.
Duchenne muscular dystrophy is a rare genetic condition characterized by progressive muscular weakness. The disease occurs due to a defective gene.
Roche Holding AG said the recent death of a patient in Brazil who had been treated with gene therapy Elevidys for Duchenne muscular dystrophy is unrelated to the treatment.
According to Roche, the boy was not a clinical trial participant. The reporting physician assessed his death as unrelated to the gene therapy. The death was reported to health authorities. Roche, which markets Sarepta's Duchenne treatment Elevidys outside the U.S., declined to comment on the boy's age or other case details.
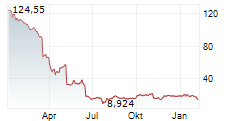
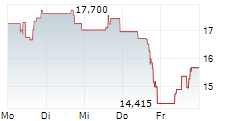
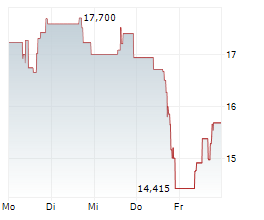
SRPT closed Friday's regular trading at $11.93 down $0.94 or 7.29%. In after-hours trading, the stock further dropped $0.46 or 3.86%.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News