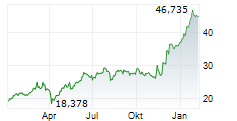
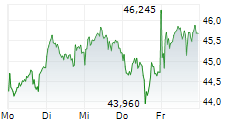
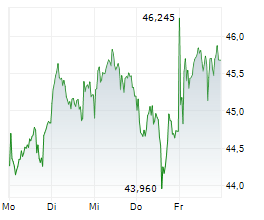
LEVERKUSEN (dpa-AFX) - Bayer AG (BAYZF.PK) on Monday said that its new drug application (NDA) for sevabertinib for the treatment of advanced non-small cell lung cancer (NSCLC) has been accepted by the Center for Drug Evaluation (CDE) of China's National Medical Products administration (NMPA).
The NDA is based on positive results from the ongoing Phase I/II SOHO-01 study of sevabertinib in patients with advanced NSCLC.
Sevabertinib has been granted priority review status by the U.S. Food and Drug Administration in May this year. The drug candidate was also granted breakthrough therapy designation by the CDE in 2024.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News