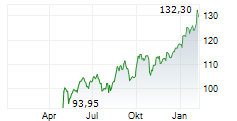
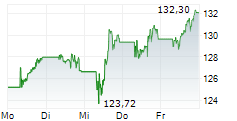
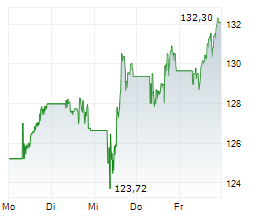
BASEL (dpa-AFX) - Novartis announced Thursday said that the US Food and Drug Administration (FDA) has approved a label update for Leqvio (inclisiran), enabling its use as monotherapy along with diet and exercise to reduce low-density lipoprotein cholesterol (LDL-C) in adults with hypercholesterolemia.
The FDA proactively requested the label update based on the robust LDL-C lowering data for PCSK9-targeting therapies.
'This first-line label update reinforces Leqvio's proven ability to effectively lower LDL-C, a critical risk factor for heart disease,' said Victor Bultó, President, US, Novartis. 'With this new indication enabling Leqvio's use as monotherapy along with diet and exercise, we now have the potential to help even more patients achieve their LDL-C lowering goals earlier in their treatment journey.'
With its twice-yearly, health care provider-administered dosing, Leqvio is uniquely positioned to help support patient adherence and long-term LDL-C management, including goal attainment.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News