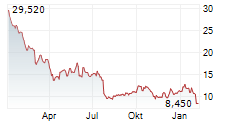
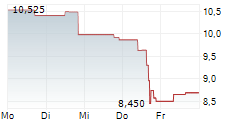
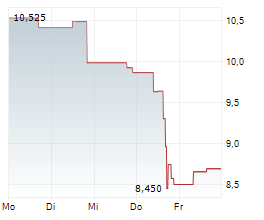
WASHINGTON (dpa-AFX) - Novocure (NVCR) Wednesday said it has submitted a premarket approval application to the U.S. Food and Drug Administration (FDA) for Tumor Treating Fields (TTFields) therapy for the treatment of locally advanced pancreatic cancer.
TTFields are a type of cancer treatment that uses electric fields to kill cancer cells.
This submission is supported by the PANOVA-3 study evaluating the use of TTFields therapy along with gemcitabine and nab-paclitaxel (GnP) as a first-line treatment for adults with unresectable, locally advanced pancreatic cancer, compared to GnP alone. The trial had met its primary goal by showing a statistically significant and clinically meaningful improvement in median overall survival for patients treated with TTFields and GnP compared to GnP alone.
Novocure expects a decision from the regulator in the second half of 2026.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News