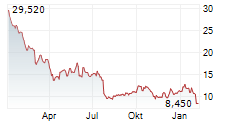
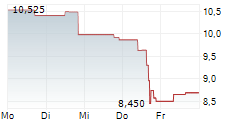
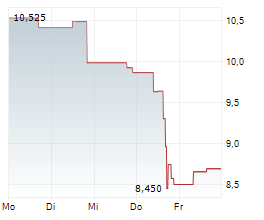
WASHINGTON (dpa-AFX) - Novocure (NVCR) announced that Japan's Ministry of Health, Labour and Welfare approved Optune Lua for concurrent use with PD-1/PD-L1 inhibitors for the treatment of adult patients with unresectable advanced/recurrent non-small cell lung cancer who have progressed on or after platinum-based chemotherapy. The MHLW approval was supported by the Phase 3 LUNAR trial.
Optune Lua is a wearable, portable medical device that produces alternating electric fields known as Tumor Treating Fields or TTFields, which are delivered through non-invasive, wearable arrays.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News