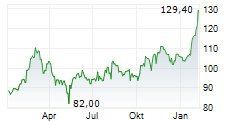
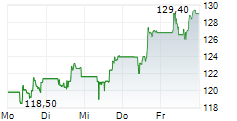
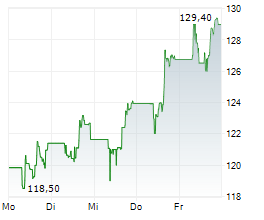
FOSTER CITY (dpa-AFX) - Gilead Sciences, Inc. (GILD) Tuesday said that the European Commission has granted marketing authorization for lenacapavir for pre-exposure prophylaxis to reduce the risk of sexually acquired HIV.
The approval was based on positive data from two Phase 3 studies dubbed PURPOSE 1 and PURPOSE 2. Data from the PURPOSE 1 study showed that twice-yearly administration of lenacapavir led to 100% reduction in HIV infections, and in PURPOSE 2 study, lenacapavir reduced HIV infections by 99.9%.
This authorization follows approval by the U.S. Food and Drug Administration (FDA) in June, as well as the issuance of guidelines by the World Health Organization (WHO) in July that recommended twice-yearly lenacapavir as an additional pre-exposure prophylaxis option for HIV prevention.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News