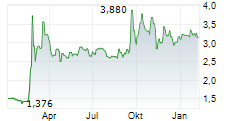
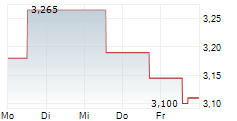
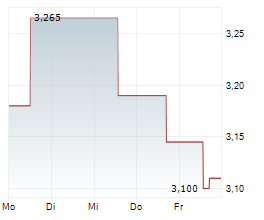
Oryzon Genomics has announced that the European Medicines Agency (EMA) has provided regulatory clearance for a Phase Ib trial of iadademstat in sickle cell disease. The Phase Ib study (named RESTORE) is expected to enrol 40 adult patients with the condition, and will be based across multiple sites in Spain. The trial is primarily designed to assess the safety and tolerability of the drug candidate, as well as to determine the recommended Phase II dose, with secondary objectives focused on measurements of foetal haemoglobin. While we acknowledge that this is an early-stage programme, we believe it represents a key milestone for Oryzon, since it will be the first investigation of iadademstat in a non-malignant haematological indication.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research