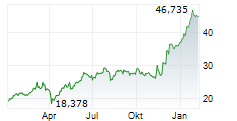
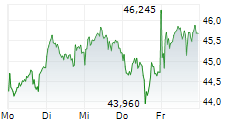
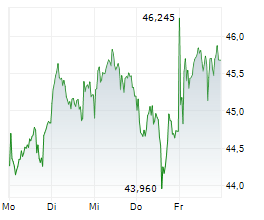
LEVERKUSEN (dpa-AFX) - Bayer AG (BYR.L) on Wednesday said that the U.S. Food and Drug Administration (FDA) has accepted its New Drug Application (NDA) for gadoquatrane, proposed for the contrast enhancement in MRI.
The submission is based on positive data from the pivotal Phase III QUANTI studies evaluating gadoquatrane in adult and pediatric patients.
If approved, gadoquatrane would become the lowest dose macrocyclic gadolinium-based contrast agent (GBCA) available in the U.S., the company added. The submitted dose of 0.04 mmol gadolinium per kilogram body weight is 60% lower than the current dose of 0.1 mmol Gd/kg body weight.
Additionally, applications for marketing authorization of gadoquatrane are under review in Japan, the European Union and other countries.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News