PETAH TIKVA (dpa-AFX) - Teva Pharmaceuticals, Inc., a U.S. affiliate of Teva Pharmaceutical Industries Ltd. (TEVA), Thursday said that the U.S. Food and Drug Administration (FDA) has approved a generic version of Novo Nordisk's Saxenda, indicated for adults with obesity.
Teva also announced U.S. launch of the drug.
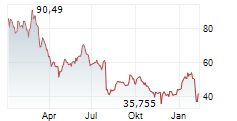
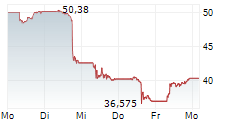
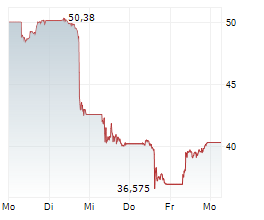
As of June 2025, Saxenda had annual sales of $165 million.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News