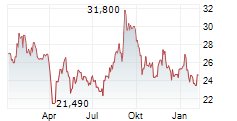
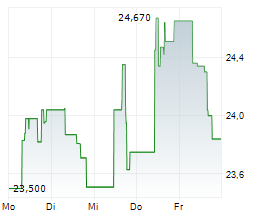
WESTON (dpa-AFX) - Eisai and Biogen (BIIB) announced that the FDA has approved the Biologics License Application for once weekly lecanemab-irmb subcutaneous injection, or LEQEMBI IQLIK, for maintenance dosing for the treatment of early Alzheimer's. LEQEMBI IQLIK will be launched on October 6, 2025 in the U.S.
LEQEMBI IQLIK is a subcutaneous autoinjector developed by Eisai, containing 360 mg/1.8 mL that can be administered in approximately 15 seconds. Eisai noted that this event will have a minor impact on the consolidated financial forecast for fiscal 2025. The company said there are no changes to the consolidated financial forecast announced on May 15, 2025.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News