| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 27,750 | 28,250 | 19:04 | |
| 27,840 | 28,200 | 27.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Do | Eisai points the way to kidney cancer support with Kompass digital hub | 3 | FiercePharma | ||
| Do | Eisai unveils digital platform for kidney cancer patients | 4 | pharmaphorum | ||
| 19.02. | Rückschlag für Alzheimer-Mittel Lecanemab von Eisai und Biogen | 533 | dpa-AFX | BERLIN (dpa-AFX) - Für den Alzheimer-Wirkstoff Lecanemab gibt es nach Ansicht eines entscheidenden Expertengremiums keinen belegten Zusatznutzen im Vergleich zu älteren Behandlungsansätzen. Der erste... ► Artikel lesen | |
| 16.02. | Eisai: Ministry of Health, Labour and Welfare Grants Orphan Drug Designation in Japan to Novel Orexin Receptor Agonist E2086 for Narcolepsy | 446 | JCN Newswire | TOKYO, Feb 16, 2026 - (JCN Newswire) - Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, "Eisai") announced today that it has received an orphan drug designation for its in-house discovered and... ► Artikel lesen | |
| EISAI Aktie jetzt für 0€ handeln | |||||
| 10.02. | Eisai: Biologics License Application for Subcutaneous Formulation of "LEQEMBI(R)" (lecanemab) for the Treatment of Early Alzheimer's Disease Designated for Priority Review in China | 534 | JCN Newswire | TOKYO and CAMBRIDGE, Mass., Feb 10, 2026 - (JCN Newswire) - Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, "Eisai") and Biogen Inc. (Nasdaq: BIIB, Corporate headquarters: Cambridge, Massachusetts... ► Artikel lesen | |
| 09.02. | Eisai Q3 FY2025 slides reveal 109% growth in LEQEMBI sales, strategic oncology investments | 11 | Investing.com | ||
| 09.02. | Eisai GAAP EPS of ¥148.31, revenue of ¥619.95B; reaffirms FY outlook | 16 | Seeking Alpha | ||
| 06.02. | Eisai, Henlius Strike Japan Commercialization Deal for PD-1 Antibody Serplulimab | 3 | Contract Pharma | ||
| 06.02. | Leqembi starts to deliver for Eisai and Biogen | 16 | pharmaphorum | ||
| 06.02. | Eisai, Henlius partner on anti-PD-1 antibody in Japan | 2 | Investing.com | ||
| 06.02. | Eisai and Henlius Enter into Exclusive Commercial License Agreement for Anti-PD-1 Antibody Serplulimab in Japan | 422 | JCN Newswire | TOKYO and SHANGHAI, Feb 6, 2026 - (JCN Newswire) - Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, "Eisai") and Shanghai Henlius Biotech, Inc. (Headquarters: Shanghai, China, CEO: Jason Zhu... ► Artikel lesen | |
| 05.02. | Eisai signs $388m deal for Japanese rights to Henlius' anti-PD-1 mAb | 3 | Pharmaceutical Technology | ||
| 05.02. | Eisai and Henlius Enter into Exclusive Commercial License Agreement for Anti-PD-1 Antibody Serplulimab in Japan | 419 | PR Newswire | TOKYO and SHANGHAI, Feb. 5, 2026 /PRNewswire/ -- Eisai Co., Ltd. ("Eisai") and Shanghai Henlius Biotech, Inc. ("Henlius") today announced the conclusion of an exclusive commercialization... ► Artikel lesen | |
| 03.02. | GHIT Fund: Total Investment of Approx. USD 8.8 Million in Malaria, Tuberculosis, and NTD R&D Projects with Partners Including Mahidol University, Barcelona Institute for Global Health, and Eisai | 1.059 | PR Newswire | TOKYO, Feb. 3, 2026 /PRNewswire/ -- The Global Health Innovative Technology (GHIT) Fund announced today a total investment of approximately JPY 1.39 billion (USD 8.8 million1) in six R&D... ► Artikel lesen | |
| 27.01. | FDA to review Eisai's Leqembi Iqlik sBLA for Alzheimer's | 13 | Pharmaceutical Technology | ||
| 26.01. | BioArctic: Eisai submits Marketing Authorisation Variation to EMA for intravenous maintenance dosing every four weeks with Leqembi (lecanemab) | 463 | PR Newswire | STOCKHOLM, Sweden, Jan. 26, 2026 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai announced today that they have submitted a proposed Marketing Authorisation... ► Artikel lesen | |
| 26.01. | Eisai and Biogen's subcutaneous Leqembi given FDA Priority Review for early Alzheimer's | 7 | PMLiVE | ||
| 26.01. | BioArctic's Partner Eisai Receives Priority Review For Leqembi Iqlik Subcutaneous Autoinjector | 356 | AFX News | TOKYO (dpa-AFX) - BioArctic AB (BRCTF) announced on Monday that its partner Eisai (ESALY) has received Priority Review from the U.S. Food and Drug Administration (FDA) for the supplemental Biologics... ► Artikel lesen | |
| 26.01. | FDA grants priority review for Eisai's Alzheimer's treatment autoinjector | 8 | Investing.com | ||
| 26.01. | Eisai: FDA Accepts LEQEMBI(R) IQLIK (lecanemab-irmb) Supplemental Biologics License Application as a Subcutaneous Starting Dose for the Treatment of Early Alzheimer's Disease under Priority Review | 631 | JCN Newswire | If approved, LEQEMBI IQLIK would be the first and only anti-amyloid treatment to offer at-home injection options for initiation and maintenance dosing for this progressive, relentless disease FDA action... ► Artikel lesen |
1 2 3 4 5 Weiter >>
133 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 42,010 | +0,39 % | Märkte am Morgen: Bayer, SAP, Scout24, Knorr-Bremse, thyssenkrupp, Bitcoin | Der DAX hat eine turbulente Woche hinter sich. Am Freitag gab es noch einmal einen ordentlichen Schub nach oben. In den USA hatte der Supreme Court die Zölle von Donald Trump für unrechtmäßig erklärt.... ► Artikel lesen | |
| NOVO NORDISK | 31,600 | -0,24 % | Rückschlag im Adipositas-Markt: Novo Nordisk verfehlt Ziel - Aktie bricht zweistellig ein! | © Foto: Blondet Eliot/ABACA - picture alliance / abacaNovo Nordisk meldet starke 23 Prozent Gewichtsverlust mit CagriSema. Doch im direkten Duell bleibt das Mittel hinter dem Rivalen zurück.Der dänische... ► Artikel lesen | |
| PFIZER | 23,350 | -0,17 % | Pfizer, Astellas Pharma Share Positive Data From Bladder Cancer Trial | NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) and Astellas Pharma Inc., Friday announced the positive results from the Phase 3 EV-304 clinical trial for PADCEV in combination with Keytruda in... ► Artikel lesen | |
| NOVARTIS | 142,44 | +0,21 % | dpa-AFX-Überblick: UNTERNEHMEN - Die wichtigsten Meldungen vom Wochenende | GESAMT-ROUNDUP 2: Kommt eine Social-Media-Altersgrenze ab 14 Jahren? STUTTGART - Ein Mindestalter für soziale Medien wie Tiktok und Instagram zum Schutz von Kindern und Jugendlichen rückt immer näher.... ► Artikel lesen | |
| MERCK KGAA | 127,75 | +1,67 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 125,66 | -0,33 % | Gilead Sciences auf Einkaufstour: Wer folgt als Nächstes? | Gilead Sciences lässt seinen Worten Taten folgen: Mit einer ersten Multi-Milliarden-Übernahme im laufenden Jahr stärkt der Pharmakonzern seine Pipeline. Weiterhin ausstehend: eine bedeutende Transaktion... ► Artikel lesen | |
| AURORA CANNABIS | 3,240 | -1,22 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| SANOFI | 81,41 | -0,40 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 25,040 | +0,93 % | GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar | DJ GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar
Von Sarah Sloat
DOW JONES--GSK hat die Übernahme des Biopharma-Unternehmens 35Pharma für 950 Millionen US-Dollar vereinbart. 35Pharma... ► Artikel lesen | |
| ABBVIE | 196,00 | -0,31 % | Rekordquartal und AbbVie-Deal beflügeln Gubra-Aktie: Kursplus von 13,88 % | ||
| ROCHE | 402,50 | -0,27 % | Hightech-Neubau für frühe Krankheitsdiagnosen: Drees & Sommer begleitete Roche bei Millionenprojekt | Penzberg (ots) - Im neu eingeweihten Diagnostik- und Innovationszentrum in Penzberg werden in Zukunft In-vitro-Diagnostika und spezifische Testverfahren entwickelt, um Krankheiten früh zu erkennen.Alzheimer... ► Artikel lesen | |
| CANOPY GROWTH | 0,950 | +0,85 % | Is It Time to Dump Your Shares of Canopy Growth?? | ||
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 889,90 | -0,11 % | Opening Bell: Coca-Cola, Nvidia, Eli Lilly, Novo Nordisk, Vistra Energy, Energy Fuels | An der Wall Street richtet sich die Aufmerksamkeit mal wieder auf die Zollpolitik von Donald Trump. Der Supreme Court hatte am Freitag die bisherigen Maßnahmen gekippt. Trump mit neuen Zöllen unter... ► Artikel lesen | |
| MERCK & CO | 104,60 | -0,19 % | Pharma im Umbruch: Milliardenblockbuster vor Patentende: Merck baut Konzern radikal um | © Foto: JHVEPhoto - stock.adobe.com Merck stellt sein Pharmageschäft neu auf. Der Konzern reagiert auf das nahende Patentende seines Kassenschlagers Keytruda und setzt auf neue Wachstumstreiber.Der... ► Artikel lesen |
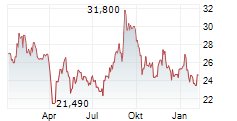
Das Unternehmen EISAI CO LTD kann der Branche Pharma zugeordnet werden. Die relative Kursveränderung beträgt aktuell -0,07 % bei einem Aktienkurs von 28,000 Euro. Beim derzeitigen Kurs ergibt sich, basierend auf einer gesamten Ausschüttung von 0,87 Euro in den vergangenen 12 Monaten, eine Dividendendrendite von +3,11 %.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu EISAI CO LTD finden Sie auf Wallstreet Online.